ETV6 Protein Could Be an Important Target for Ewing Sarcoma Treatment
, by Linda Wang
Ewing sarcoma is an aggressive childhood cancer that is particularly difficult to treat, and little progress has been made in developing effective therapies for patients with disease that has spread or come back after treatment. That lack of progress has been linked, in part, to the challenges in developing drugs that can block the activity of a fusion protein known as EWS-FLI1, which is responsible for the vast majority of Ewing sarcoma tumors.
Now, using genetic screening, two research groups—one led by Kimberly Stegmaier, M.D., of Dana-Farber Cancer Institute, and the other by Christopher Vakoc, M.D., Ph.D., of Cold Spring Harbor Laboratory—have independently discovered that a protein called ETV6 also appears to play a critical role in Ewing sarcoma.
Both groups found that ETV6 indirectly promotes tumor growth by modulating EWS-FLI1’s behavior. In experiments in cancer cells and mice, the groups showed that, in the absence of ETV6, EWS-FLI1 goes into overdrive. But rather than revving up tumor growth even further, EWS-FLI1’s extreme hyperactivity halts tumor growth.
The researchers hope these new insights can be used to develop a targeted drug that interferes with the interaction between ETV6 and EWS-FLI1 and might lead to an effective treatment for Ewing sarcoma.
Both studies were published January 19 in Nature Cell Biology.
“The fact that this discovery came independently out of the two labs is rock-solid validation that this is something worth pursuing,” said Keren Witkin, Ph.D., of NCI’s Division of Cancer Biology, who was not involved in either study. “It's a nice step forward, because it gives us a new place to look for potential treatment strategies.”
What’s more, Dr. Witkin continued, these studies have helped researchers gain a clearer picture of what’s going on at the molecular level in Ewing sarcoma, which may provide clues to what’s going awry in other childhood cancers fueled by fusion proteins.
Expanding the search for drug targets
Ewing sarcoma is a rare cancer that forms in bone or in the soft tissue surrounding bone. It’s most commonly diagnosed in adolescents and young adults and accounts for about 2% of all childhood cancers.
The disease is typically treated with chemotherapy, surgery, and radiation. When the cancer is diagnosed before it has spread, five-year survival rates are about 70%. Survival rates are much lower when the disease has spread or come back after treatment.
And even when patients go into remission, there are often lasting effects from chemotherapy and radiation, including significant disabilities and the increased risk of second cancers. So researchers have focused on finding ways to attack the cancer cells directly while minimizing harm to healthy cells.
EWS-FLI1 is a transcription factor, meaning it is directly involved in the process of gene expression, and it regulates multiple genes that drive Ewing sarcoma. As is often the case with transcription factors, researchers have struggled to develop drugs that can lock on to EWS-FLI1 and reverse its effect on gene expression.
As a potential workaround, researchers have searched instead for ways to indirectly interrupt the fusion protein’s activity—namely by disrupting other proteins that EWS-FLI1 relies on to carry out its functions. One approach to finding such proteins involves a genetic screening technique called CRISPR-Cas9.
In 2021, using CRISPR-Cas9, Dr. Stegmaier’s team at Dana-Farber found that an enzyme called TRIM8 appears to play a key role in the growth of Ewing sarcoma. TRIM8, they showed, is involved in degrading EWS-FLI1, a process that helps to maintain precise levels of the fusion protein that tumors need to grow. Without TRIM8, levels of the fusion protein increase, which is toxic to Ewing sarcoma cells.
“It’s fascinating biology,” Dr. Stegmaier said. “A lot of what we've learned about Ewing sarcoma and EWS-FLI1 recently is related to the ‘Goldilocks phenomenon.’ Precise amounts of the fusion protein are needed to initiate and maintain the disease. Too much or too little of the EWS-FLI1 protein is toxic to Ewing sarcoma cells.”
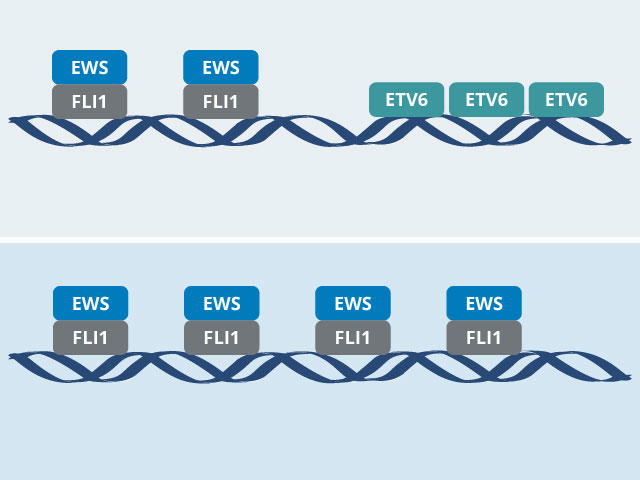
In the new studies, both teams used CRISPR-Cas9 to systematically identify genes that Ewing sarcoma cells, but not normal cells, rely on for their survival. They both landed on the ETV6 gene and, through additional experiments, discovered why this protein is important in Ewing sarcoma: The ETV6 protein is in constant competition with the EWS-FLI1 fusion protein, each vying to bind to specific segments of DNA where key genes involved in cell growth and survival reside.
“ETV6 is keeping EWS-FLI1 in check on DNA, and that is an important mechanism for tumor growth,” said Diana Lu, Ph.D., of Harvard Medical School, who conducted the ETV6 studies when she was a graduate student in Dr. Stegmaier’s lab.
“One protein turns the DNA off, and the other one turns it on. And that turns out to be the key to this molecular mechanism that we unpacked in the study,” Dr. Vakoc said.
As a transcription factor, ETV6 ultimately may present the same challenges to researchers as EWS-FLI1: developing drugs that can effectively bind to it.
However, Dr. Vakoc’s team created a peptide, or small chain of amino acids, that interferes with a specific region of the ETV6 protein that is essential to its functioning. In their study, when they introduced this peptide to Ewing sarcoma cells, the cells stopped growing and began taking on properties of normal cells.
Implications for other childhood cancers
Dr. Witkin noted that these studies demonstrate the important role that genetic screening with tools like CRISPR-Cas9 can play in identifying new drug targets.
“In Ewing sarcoma, we know the driver, but we haven't had much success targeting it. In cases like this, the CRISPR-Cas9 screening approach really enables you to find something else essential for the cancer’s development and progression and give you potential drug targets,” she said.
This work, she continued, was supported by the Fusion Oncoproteins in Childhood Cancers (FusOnC2) Consortium. FusOnC2 is a Cancer MoonshotSM-supported initiative launched by NCI to bring researchers together to better understand the biology of fusion proteins that play an important role in driving childhood cancers.
Dr. Stegmaier thinks there could be other targets for Ewing sarcoma that will be uncovered as screening technology advances. She noted that they conducted most of their CRISPR-Cas9 library screening in lab-grown cells, which can behave differently than cells in the complex metabolic environment of a living organism. Genetic screening of animal models could help researchers identify targets that may have been missed in lab-grown cells, she said.
From a bigger picture perspective, the delicate interplay between EWS-FLI1 and ETV6 in driving tumor growth may not be unique to Ewing sarcoma, Dr. Witkin said.
“This interesting mechanism of competition between transcription factors could be relevant in other [childhood] cancers as well,” she said. For example, researchers at the University of Michigan in Ann Arbor recently found that ETV6 may affect the behavior of an important transcription factor in the most common form of leukemia in children.
Meanwhile, Dr. Stegmaier and Dr. Vakoc are discussing collaborative projects to translate their findings into new treatments for Ewing sarcoma that may point to treatments for other childhood cancers driven by fusion proteins like EWS-FLI1.
“No one should give up on trying to make drugs [that target] EWS-FLI1 [directly], but now there are other candidates,” Dr. Vakoc said.