Antibody Drug Ejects Problematic Proteins from Cancer Cells
, by Nadia Jaber
Antibodies are used in many kinds of cancer treatments but have only been able to reach proteins on the outside of cancer cells—until now. According to a new study, scientists have designed antibodies that can barge into cancer cells and drag abnormal proteins out, ultimately slowing tumor growth in mice.
The approach is a novel way of targeting cancer-fueling proteins that are buried inside cancer cells, several experts said.
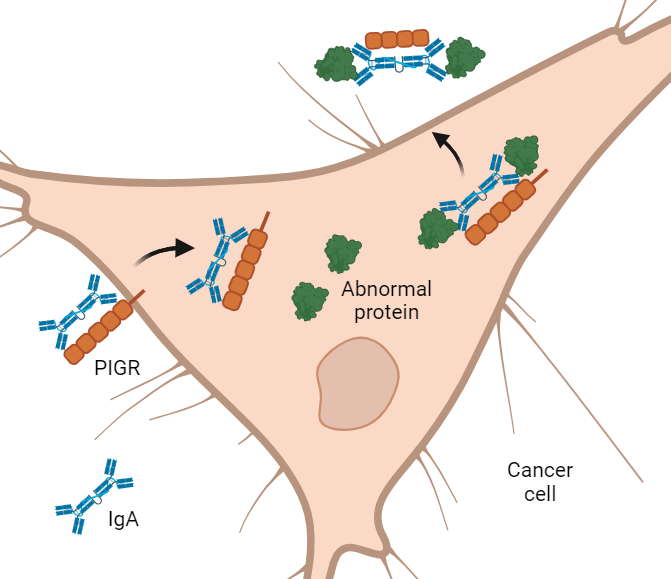
Most antibodies can’t get inside cells. Yet most cancer-fueling proteins are tucked deep inside cancer cells. One type of antibody, however, called IgA, can glide through certain cells like a ghost walking through a wall.
In the NCI-funded study, researchers designed IgA antibodies that get into cancer cells and latch onto abnormal proteins that drive cancer growth. As the antibodies exit the cells, they pull the mutated proteins out with them like bouncers throwing unruly patrons out of a bar.
One of the antibodies targets an abnormal form of a protein called KRAS. Abnormal KRAS proteins are present in more than 30% of cancers and, until recently, were nearly impossible to target with traditional chemical-based drugs.
In mice, treatment with the KRAS-targeting antibody substantially slowed the growth of tumors with mutant KRAS proteins, according to results published October 30 in Immunity.
The study findings suggest that “we should be able to target basically any protein in the cytoplasm of a tumor cell for which we have a specific dimeric IgA antibody,” said the study’s leader, José Conejo-Garcia, M.D., Ph.D., of Duke School of Medicine.
The work, part of the Cancer MoonshotSM Immuno-Oncology Translational Network, is a “super exciting, extremely novel approach using IgA antibodies,” said Kevin Howcroft, Ph.D., of NCI’s Division of Cancer Biology, a coordinator of the Network.
IgA targeting mutant KRAS
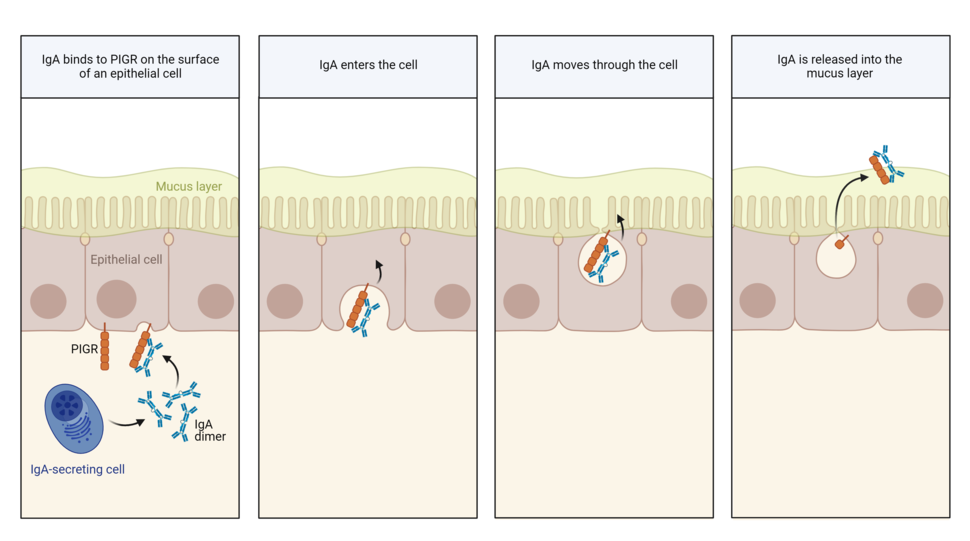
IgA’s normal job is to prevent germs and toxins from breaching the protective lining of hollow organs like the nose, gut, and uterus. It stands guard in the mucus covering the inner cavity of these organs, where it neutralizes potential invaders.
IgA is made by certain immune cells. To reach the mucus layer of hollow organs, IgA must move across epithelial cells lining these organs. To do so, it grabs onto a handle called PIGR on the surface of epithelial cells. PIGR then carries it through the cells and spits it into the mucus layer on the other side.
As IgA passes through epithelial cells lining the gut, it has been shown to scoop up and drag out viruses, Dr. Conejo-Garcia explained.
“So, we thought, can we use this mechanism to clear abnormal proteins inside cancer cells?” he said.
To test that idea, the researchers created IgA antibodies that latch onto a mutant protein known as KRAS-G12D, one of the most commonly mutated proteins in cancer.
When the researchers mixed the KRAS-targeting antibody with lung or ovarian cancer cells grown in dishes, they saw that the antibody slipped inside the cells. And as it passed through the cells, it snatched up abnormal KRAS proteins and pulled them out.
In mice with human lung or ovarian tumors with mutant KRAS, treatment with the IgA antibody substantially slowed tumor growth and didn’t cause any obvious side effects. An experimental KRAS-G12D–blocking drug being tested in a clinical trial also slowed tumor growth in the mice, but the IgA antibody was more effective.
Help from cancer-killing T cells
In a previous study, Dr. Conejo-Garcia and his colleagues found that when IgA passes through ovarian cancer cells, it makes those cells an easier target for cancer-killing T cells.
That also appeared to be true for the KRAS-targeting IgA antibody. The researchers found that T cells were partially responsible for slowing tumor growth in antibody-treated mice.
Like some antibody-based cancer treatments, the researchers think that IgA may coat cancer cells, alerting the immune system that the cell is abnormal and should be destroyed.
That could mean that future IgA-based antibody treatments might work well in concert with immunotherapies such as immune checkpoint inhibitors, which help T cells recognize and attack cancer cells, the scientists noted.
Although the IgA-based antibodies look promising on their own, Dr. Howcroft said, in the future, they are more likely to be used in combination with other cancer treatments.
Getting IgA deeper inside cancer cells
Mutant KRAS proteins tend to hang out near the cell membrane, which might make it relatively easy for IgA antibodies to grab onto the proteins as they enter cancer cells, Dr. Conejo-Garcia explained.
But many cancer-fueling proteins reside deeper in the cell, in the cytoplasm. The researchers wondered, could IgA antibodies also target these proteins?
To find out, they designed an IgA antibody that targets an abnormal form of IDH1, a protein that is located in the cytoplasm. Abnormal IDH1 proteins drive the growth of some brain cancers and, less frequently, some colon and lung tumors.
In mice with colon tumors that have mutant IDH1, the antibody slowed tumor growth even though the tumors had low levels of PIGR, the handle IgA uses to get into cells. When the scientists boosted levels of PIGR in colon tumors, the antibody treatment slowed tumor growth even more.
The research team is currently working on ways to get IgA antibodies even deeper into the cell—namely, into the nucleus.
If they are successful, it might be possible to target proteins called transcription factors, Dr. Conejo-Garcia noted. These proteins fuel the growth of many cancers and have proven very difficult to block with traditional drugs.
IgA antibodies may breach multiple tumor types
To investigate whether IgA might be able to pass through other cancer types, the researchers looked at PIGR levels in thousands of human tumor samples.
They found high levels of PIGR in tumors derived from epithelial cells such as kidney, uterine, and endometrial tumors.
“We have found that this is probably a universal phenomenon” in epithelial tumors, Dr. Conejo-Garcia said, referring to IgA’s ability to pass through cancer cells. However, he added, IgA likely does not travel through non-epithelial tumors such as melanoma and glioblastoma, which have low levels of PIGR.
“Epithelial carcinomas account for 80% to 90% of all cancer diagnoses,” noted Stuart Prince and Maija Hollmén, of the University of Turku in Finland, in an accompanying editorial on the study.
In addition to potentially having wide applicability, IgA-based antibody treatments could also have substantial versatility, Dr. Howcroft said. It's possible that IgA antibodies could be engineered to have different activities or to carry cargo directly into cancer cells to destroy them, he explained.
But there's still more to learn about precisely how these IgA antibodies work, Dr. Howcroft added, including how they interact with their target proteins inside cancer cells and what happens to the IgA-target protein complexes once they are tossed out of cancer cells.