Liquid Biopsies on the Horizon for Children with Solid Cancers
, by Elia Ben-Ari
Cancer researchers continue to make progress in developing tests using liquid biopsies that could complement and even serve as an alternative to traditional tissue biopsies. These tests, which analyze bits of free-floating genetic and other material shed by tumors into the blood and other body fluids, are already being used to detect cancer-related genetic changes and guide treatment decisions.
Until recently, much of the research on liquid biopsies focused on their use in adults with cancer. But scientists have begun to make progress in developing liquid biopsy tests specifically for use in children with cancer, including those with solid cancers like Ewing sarcoma, osteosarcoma, and Wilms tumor, a type of kidney cancer.
A recently published study by researchers at Children’s Hospital Los Angeles (CHLA) and the University of Southern California describes the use of one such liquid biopsy approach.
The test, which analyzes bits of DNA in samples of blood, showed promise in diagnosing various types of solid tumors in children, the team reported February 20 in npj Precision Oncology. It also showed potential for determining whether a tumor is responding to treatment or may be coming back after initial successful treatment.
“It’s a very well done, interesting study and adds a lot of data to the field,” said Mark Applebaum, M.D., a pediatrics researcher at the University of Chicago, who noted that other teams have developed similar approaches. Dr. Applebaum also works in this area but was not involved in the new study.
Liquid biopsies are of particular interest for use in children because they are less invasive and easier to repeat than tissue biopsies, said Jaclyn Biegel, Ph.D., director of the Center for Personalized Medicine at CHLA, who co-led the new study.
The new findings provide “proof of principle” that the test could be useful in the clinic, said Brian Sorg, Ph.D., a program director in NCI’s Cancer Diagnosis Program, who also was not involved in the study.
Challenges of performing biopsies in children
Accurately diagnosing cancer in children can be difficult because children can develop a wide range of solid tumors in different parts of the body. And each tumor type often has multiple subtypes with different features that can affect a patient’s prognosis and treatment, said Miguel Ossandon, Ph.D., also of NCI's Cancer Diagnosis Program.
A tissue biopsy, in which a sample of tumor is removed with a large needle or during surgery, is still the gold standard for diagnosis, Dr. Ossandon said. “But tissue biopsy has limitations, and some of those limitations can be overcome with a liquid biopsy.”
Some liquid biopsies developed for use in adults with cancer have been approved by the Food and Drug Administration to help guide treatment decisions. But liquid biopsies developed for adults can’t be used for children with cancer.
That’s because “the tumor types you see in children are very different from what you see in adults—the tumors are driven by different genomic [changes],” Dr. Biegel said.
In addition, she said, pediatric liquid biopsies must work with smaller samples of blood or other body fluids.
Liquid biopsy looks for genetic changes typical of pediatric cancers
The liquid biopsy approach that Dr. Biegel and her colleagues developed “is geared towards using very small amounts of DNA” in blood and other body fluids to look for genetic changes typically found in pediatric cancers, she said.
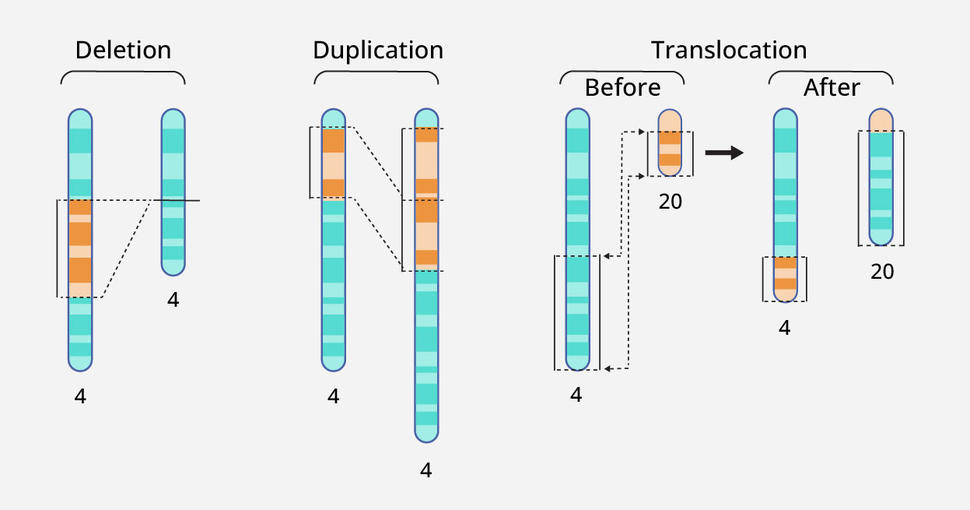
Cancer in adults often results from small changes, or mutations, in one or a few letters (nucleotides) of the DNA code in cells. But solid cancers in children are often caused by changes in the structure of chromosomes in cells, Dr. Biegel explained.
These changes can include the disappearance or duplication of one or more genes in a chromosome, known as copy number alterations. Another common change is a gene fusion, which can result when part of the DNA from one chromosome moves to another chromosome, joining parts of two different genes.
The liquid biopsy test looks for copy number alterations using a technique called low-pass whole-genome sequencing. This method, which can be applied to many samples simultaneously at relatively low cost, can also detect some but not all single-nucleotide changes.
And whereas many liquid biopsies being developed for use in children are designed to detect a single type of cancer, the CHLA and USC team’s approach, as well as similar tests being developed by other researchers, is designed to detect multiple cancer types.
Initial evaluation yields promising results
To evaluate whether their low-pass whole-genome sequencing method could identify the presence of cancer in children known to have the disease and distinguish those with cancer from those without cancer, the team analyzed blood samples from 73 patients with a variety of solid cancers and 19 patients who did not have cancer (controls).
Patients ranged in age from 6 months to 28 years. All cancer patients in the study had a diagnosis that was previously confirmed using established testing methods.
The test successfully detected copy number alterations in DNA from the blood of patients with a range of pediatric solid cancer types, including in 26 of 37 newly diagnosed patients (70%). Of those patients, 27 had localized cancer—that is, the cancer had not spread beyond its original location in the body—and copy number alterations were found in 18 (67%) of them.
The ability to detect copy number alterations in samples from children with localized cancer is noteworthy, Dr. Biegel and her team wrote, because the levels of tumor DNA circulating in blood tend to be lower in patients with localized (earlier-stage) disease.
Of the 19 patients who did not have cancer, 16 did not have detectable copy number alterations. The other three had hereditary losses of a portion of one chromosome that were not related to cancer and were therefore dropped from further analysis.
Next, the team looked at whether their test could detect specific patterns of copy number alterations that are linked with individual cancer types. The test’s ability to do so, they reported, varied depending on the type of cancer.
The researchers also wanted to see if the test has potential to track how childrens’ cancers are responding to treatment or enable earlier detection of cancer that has come back (recurred) after treatment is completed. So, using blood samples taken from patients at different times after treatment, they looked for changes in the levels and patterns of copy number alterations.
In some children, copy number alterations could no longer be detected following treatment, suggesting that the test could be used to monitor patients’ responses to treatment. And in one patient with advanced osteosarcoma, the test detected copy number alterations in blood samples—indicating that the cancer had come back—taken 9 months before imaging tests had shown a recurrence of the cancer.
Since completing this study, Dr. Biegel said that after additional testing, her team has begun using their test for copy number alterations as part of patient care at CHLA and “it’s been extremely helpful,” she said.
For instance, in one patient they used the liquid biopsy to distinguish between two types of childhood liver cancer, hepatoblastoma and a rare cancer called embryonal sarcoma of the liver, that have very different treatments and outlooks.
The team also used the test in samples of cerebrospinal fluid (fluid that circulates around the brain) from some children with brain cancer to help identify cancer that remained after treatment when imaging tests were inconclusive, Dr. Biegel said.
Next version: Additional types of cancer-related changes
The next version of the liquid biopsy test will also look for individual mutations and gene fusions that are well-established markers of certain pediatric cancers using a method known as targeted sequencing. In contrast to low-pass whole-genome sequencing, this method is a deeper dive into one specific region of the genome and more reliably detects these types of genetic changes.
To see if they could detect these changes with a liquid biopsy as effectively as they could using tumor samples, the researchers tested their targeted sequencing approach on blood samples from children with Ewing sarcoma or alveolar rhabdomyosarcoma. They were able to detect cancer-related gene fusions in most patients, including some who did not have detectable copy number alterations.
Both the low-pass whole-genome sequencing and targeted sequencing tests can be done at the same time on a single small sample of blood or other body fluid, Dr. Biegel said.
And, Drs. Ossandon and Sorg said, combining these two tests could improve the performance of the liquid biopsy for detecting and diagnosing childhood cancers.
However, further studies are needed to figure out exactly how and in what cases the combination of these two types of genetic analysis will be most useful, Dr. Biegel said.
She also noted that their liquid biopsy method is not intended as a standalone test but is meant to be combined with other diagnostic methods, such as imaging, when a tissue biopsy is not feasible.
Indeed, like other diagnostic tests, liquid biopsies have both advantages and disadvantages. For instance, Dr. Sorg said, a liquid biopsy can capture genetic material shed by tumors throughout the body, whereas a tissue biopsy “only tells you about the portion of the specific tumor that you’re sampling.”
On the other hand, he continued, a liquid biopsy “doesn’t tell you anything about where the tumors are located,” but that information can usually be obtained with imaging tests.
Before the new test is ready for wider use, Dr. Ossandon said, it will need to be validated in more patients, including children who do not yet have a definitive cancer diagnosis. In addition, he said, clinical trials that follow patients over time will be needed to find out if this and other pediatric liquid biopsy approaches can be used to monitor cancer effectively or provide accurate information on a patient’s prognosis.
“Using low-pass whole-genome sequencing to detect copy number aberrations and [look at] patterns of change over time is a very feasible and doable concept,” Dr. Applebaum said.
“However, we’re still trying to figure out how to use it to guide therapy.”