Stress-Induced Immune Changes May Help Cancer Spread
, by Sharon Reynolds
Decades of research have established that chronic stress—from money worries, job problems, family tensions, or other sources—causes chemical changes in the body. These changes can contribute to multiple problems like increased blood pressure, inflammation, and the release of certain hormones.
Some studies have suggested that these stress-induced changes can increase the risk of conditions like heart disease and diabetes, and even cancer or the spread of cancer.
In a new study, researchers have now identified biological changes induced by stress that may help explain how it could cause a tumor to spread, or metastasize, elsewhere in the body.
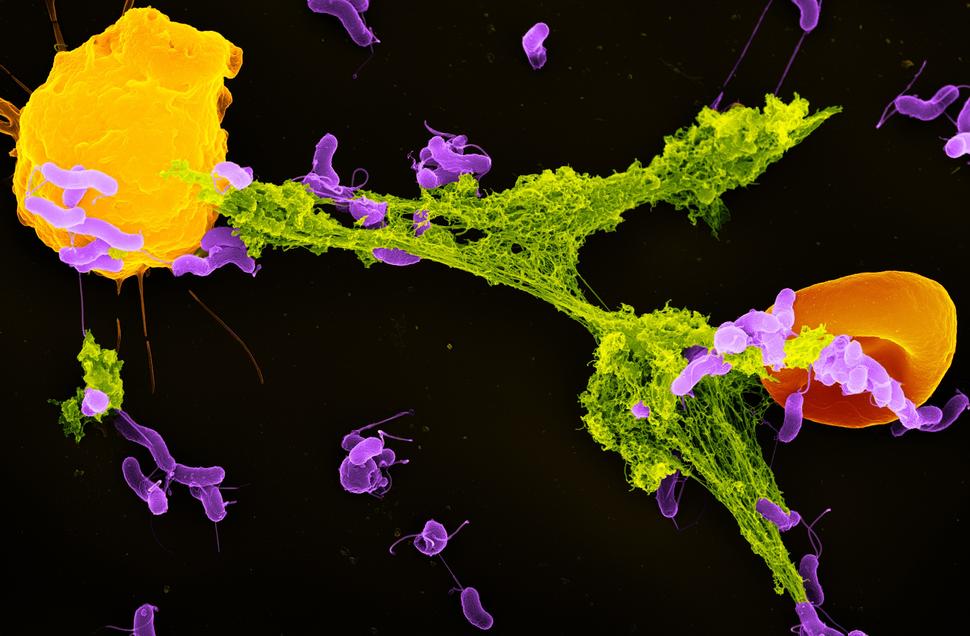
In the study, the researchers showed that stress-induced hormones called glucocorticoids can cause changes in immune cells called neutrophils. In experiments in mice, chronic exposure to glucocorticoids caused neutrophils to overproduce structures called neutrophil extracellular traps, or NETs. And these sticky traps contributed to an environment that helped metastatic cancer cells grow in distant organs.
When the researchers used compounds that broke up NETs in stressed mice with mammary tumors, the tumors were much less likely to spread to their lungs, they reported February 22 in Cancer Cell.
“When people think about tumors, they think about the cancer cells themselves. But a tumor [contains] many other types of cells that can promote cancer growth,” said Xue-Yan He, Ph.D., of Washington University School of Medicine in St. Louis, who co-led the study with Mikala Egeblad, Ph.D., now at Johns Hopkins University.
“We need to understand how the environment in distant tissues can be changed, to switch it from supporting metastases to suppressing them,” Dr. He added.
"This is an exciting study, but more research is needed to move this idea towards testing in clinical trials," said Joanna Watson, Ph.D., of NCI’s Division of Cancer Biology, who was not involved with the study.
"But right now, identifying how stress impacts cancer patients biologically draws attention to the importance of managing stress and providing more comprehensive mental-health care for cancer patients," added Brunilde Gril, Ph.D., also of the Division of Cancer Biology.
Paving the way for metastasis
Stress is unavoidable in modern life. One class of hormones produced by the body in response to stress, called glucocorticoids, can potentially influence almost every cell type in the human body. Because of their widespread impacts, overexposure to glucocorticoids has been linked to a wide range of health problems.
Prior studies have found links between glucocorticoids and the risk of cancer metastasizing. Several years ago, Dr. He was part of a team led by Dr. Egeblad at Cold Spring Harbor Laboratory in New York that began to look at the bigger picture of how this might happen, from exposure to a stressful environment to the eventual formation of a metastatic tumor.
To conduct the study, the researchers used two established methods for modeling stress in mice. One is designed to mimic exposure to constant, low-level, predictable stress. The other simulates intermittent, unpredictable, mild stress.
They used these methods to induce chronic stress in two different mouse models of breast cancer. In both models, when the mice were exposed to stress using either method, they had both larger mammary tumors and more lung metastases than mice not exposed to stress.
But a series of follow-up experiments strongly suggested that this increased tumor growth and metastasis wasn’t being driven by the effects of stress on cancer cells themselves.
So the researchers turned their attention to the next logical culprit, the other cells and structures around metastatic tumors that formed in their lungs, known as their microenvironment.
A sticky home for metastatic cells
Genetic analyses of lung tissue from stressed mice revealed telltale signs that stress was causing changes in lung tissue—including changes that can block immune cells from killing diseased cells—that made the tissue a particularly hospitable spot for metastatic tumors.
The team next looked at how the same methods of inducing stress affected the immune cells found in lung tissue. Although they found fewer cancer-fighting T cells in the lungs of stressed mice, their experiments indicated that this reduction wasn’t being driven by the direct effects of glucocorticoids on the T cells.
But glucocorticoids did seem to alter the activity of a type of immune cell called neutrophils. Exposure to stress or directly injecting glucocorticoids into mice caused neutrophils to migrate into their lung tissue. The neutrophils, in turn, coaxed another type of cell to produce a protein called fibronectin, which is known to promote a hospitable environment for metastatic cells.
When the researchers removed glucocorticoid-induced neutrophils from mice, stress exposure no longer increased the formation of metastatic tumors in their lungs.
Closer examination of stress-induced neutrophils found many concerning changes from normal neutrophil behavior, including a propensity for producing an overabundance of NETs, which are a meshwork of DNA and toxic enzymes that normally trap and kill invading germs. But it’s also known that NETs can provide an environment conducive to metastatic cancer growth.
Neutrophils from stressed mice form more NETs than those taken from unstressed mice, the researchers found. And stressed mice, or those directly given glucocorticoids, had more NETs in their bloodstream and lungs than unstressed mice, Dr. He said.
Knocking out NETs
Their next step was to see if they could block NET production in neutrophils grown in the lab. Those experiments confirmed that several different types of drugs could indeed disrupt NET production in neutrophils that had been exposed to glucocorticoids.
Finally, Dr. He and her colleagues tested what would happen to lung metastases if they got rid of NETs in mice.
They treated stressed mice with mammary tumors with a compound called DNase I that can block NET formation. The treatment reduced lung metastases compared with stressed, untreated mice. It also reduced metastases to the spleen in mice with pancreatic tumors.
To date, no studies have linked stress, glucocorticoid levels, and NET formation with survival in people with cancer. So Dr. He and her team performed preliminary studies using tumor samples that had been collected from people with breast cancer.
They first identified a set of genes in mice whose activity, or expression, changed in response to chronic stress exposure. Then, they looked for similar gene expression changes in tumor samples taken from people with breast cancer.
Not only did they find similar patterns of gene expression in some of the tumor samples, but they also showed that people with hormone receptor–positive breast cancer whose tumors had these patterns of gene expression didn’t live as long as people whose samples lacked them. However, they did not see that same association in people with hormone receptor–negative or HER2-positive tumors.
Some of the drugs the team used to shut down NET production in their experiments in mice, including CDK4/6 inhibitors, are FDA approved to treat hormone-receptor positive metastatic breast cancer. But more studies are needed to evaluate NET formation in people before thinking of using this strategy to prevent metastases, explained Dr. Gril.
“This study was very rigorously done in mice. But we need to know if this NET formation actually happens in patients due to stress” before targeting NETs with drugs, she explained.
Another important caution, added Dr. He, is that the scenarios tested in this study are very different from the short-term exposure to synthetic glucocorticoids that many people with cancer currently get. Synthetic glucocorticoids such as dexamethasone are commonly used briefly during cancer treatment to help combat side effects of chemotherapy.
“We’re not saying ‘don’t use glucocorticoids in the clinic,’ because they’re very effective for reducing side effects from treatment,” said Dr. He.
In these cases, the duration of exposure likely matters, agreed Dr. Gril. “Chronic stress can be for months or years. That’s different from an injection of dexamethasone.”
Asking about and treating stress
While the changes to the immune system uncovered in this study may be one way that stress drives metastasis, it’s unlikely to be the only way, Dr. He said. Her team is now looking at whether dysfunction in the nervous system caused by excess glucocorticoids might also help cancer cells spread.
Other immune cells may also be culprits in different cancer types. “We found that neutrophils are critical in driving the spread of cancer to the lungs. But different organs have different environments, with different immune cells playing different roles,” said Dr. He. “We don’t [yet] know whether glucocorticoids [affect] other types of immune cells.”
The many potential ways that stress might drive metastasis points to the importance of recognizing and treating stress in patients, explained Dr. Gril.
Existing patient-reported outcome surveys “are effective at evaluating levels of stress,” she said.
“And there are more and more psychological interventions now, such as cognitive behavioral therapy, to help patients cope with stress and improve their quality of life. So this is something we need to be asking patients about as part of their care.”