Pembrolizumab Shows Promise in Patients with Rare Form of Skin Cancer
, by NCI Staff
In a small clinical trial, more than half of the patients with an aggressive form of skin cancer called Merkel cell carcinoma responded to the drug pembrolizumab (Keytruda®), which strengthens the immune response against cancer. Overall, the responses have been longer-lasting than those typically seen in patients with this very rare cancer who have received chemotherapy.
The 26 patients in this phase II trial—which had funding support from NCI—had an advanced form of the disease, and none had received prior systemic treatment. Among the 25 patients who could be evaluated, 14 patients (56 percent) had a complete or partial response, Paul Nghiem, M.D., Ph.D., of the Fred Hutchinson Cancer Research Center reported on April 19 at the annual meeting of the American Association for Cancer Research (AACR) in New Orleans.
“It was clear very early in the trial that a strong fraction of patients responded really well to the treatment and, just as important, that their responses appear to be durable,” said Dr. Nghiem, who also holds a position at the University of Washington School of Medicine. “This study is one of the first to demonstrate the concept that virus-driven cancers may be responsive to immunotherapy.”
The findings were published simultaneously in the New England Journal of Medicine.
“This [trial] is very exciting, and these result are very meaningful,” said Raymond N. DuBois, M.D., Ph.D., of the Medical University of South Carolina College of Medicine, who moderated a press briefing about the research at the AACR meeting.
About 80 percent of Merkel cell carcinomas are associated with infection by a virus called Merkel cell polyomavirus (MCPyV). The other major risk factor is exposure to ultraviolet rays of the sun, which can damage cells found in the top layers of the skin. When cancer arises among certain skin cells, it can take on the look of normal Merkel cells, whose roles include mediating touch sensation. Compared with malignant melanoma, Merkel cell carcinoma tends to spread more aggressively and is several times more likely to be lethal than melanoma.
No drugs have been approved by the Food and Drug Administration for the treatment of Merkel cell carcinoma. Some patients respond to chemotherapy, but the disease typically progresses after about 3 months, leaving patients with few, if any, treatment options.
Potential Implications for Other Cancers
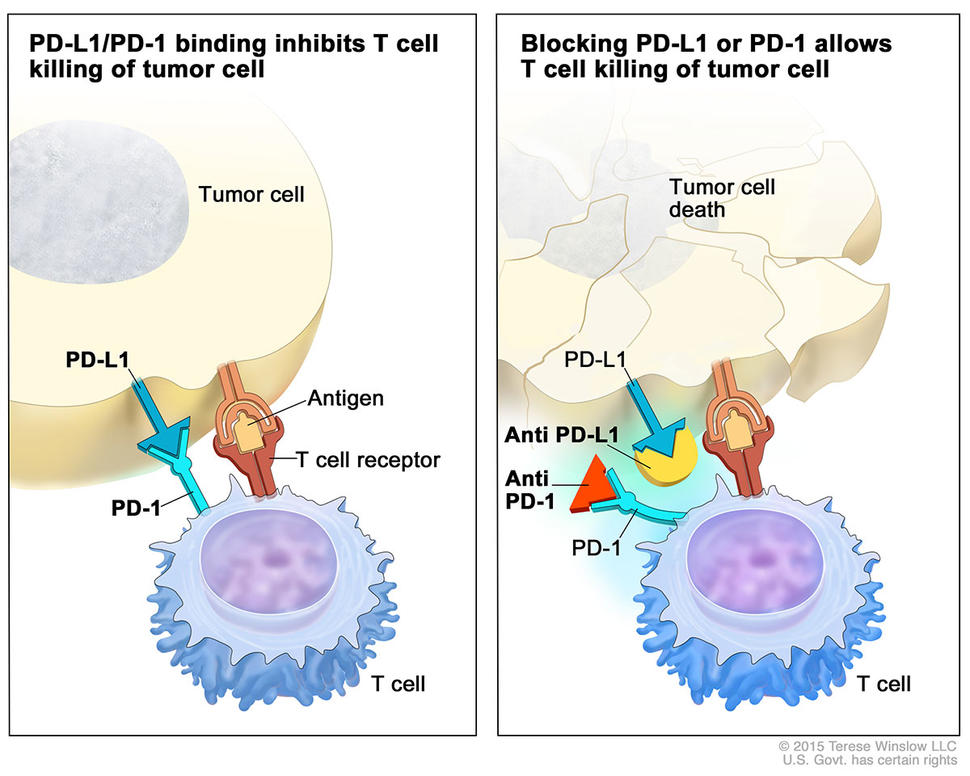
Pembrolizumab, which is approved to treat melanoma and certain types of lung cancer, targets a protein called PD-1 that restrains immune system activity. The drug, one of a class of therapies known as immune checkpoint inhibitors, removes this brake on the immune system, allowing certain immune cells to attack tumors.
In the study, the responders included patients whose tumors tested positive for MCPyV as well as patients whose tumors did not. More research will be needed to understand the mechanisms by which the drug works in these different types of tumors, the study authors said.
“This is a relatively small study, but the results are very promising,” said coauthor Suzanne Topalian, M.D., of the Sidney Kimmel Comprehensive Cancer Center. “And, although the study was about a rare disease, the results may be emblematic of the use of immunotherapy for a much larger group of cancers—those that are associated with viral infections.”
Worldwide, about 20 percent of cancers are associated with infectious agents such as viruses. Persistent infection with certain types of human papillomaviruses, for example, can lead to cervical and other types of cancer.
The Merkel cell carcinoma study raises the question of whether other viruses that are associated with cancer produce antigens that stimulate the immune system to reject a tumor, Dr. Topalian explained. “It’s an open question, but a number of clinical trials are investigating it.”
Exploring Next Steps
The researchers will be expanding the current trial, adding about two dozen more patients. This could help them better understand important questions, such as which patients are most likely to respond to immunotherapy and how long patients should be treated after responding.
“We also need to learn how to combine this treatment with other therapies to benefit the patients who do not respond to the immunotherapy alone,” said Dr. Nghiem.
The side effects of pembrolizumab were manageable for most patients, he continued. But two patients in the trial had to stop treatment after only one or two infusions because of side effects. Nearly a year later, however, both of those patients had ongoing tumor responses.
“For these two individuals, the immune system is clearly doing the job, at least for now,” said Dr. Nghiem. “This suggests that it may be possible to just poke the immune system—to wake it up—and then see a persistent benefit. This is an exciting idea to consider.”
Building a Partnership
Dr. Nghiem initially had difficulty attracting a major pharmaceutical company to test an immune-based therapy for patients with Merkel cell carcinoma because the disease is so rare— approximately 2,000 cases are diagnosed each year in the United States.
Eventually, support from NCI’s Cancer Therapy Evaluation Program (CTEP) and the Cancer Immunotherapy Trials Network allowed the investigators to develop the clinical trial in collaboration with Merck, the maker of the drug, and multiple clinical sites.
“This trial is a good example of how NCI can work with partners in academia and industry to bring an important new therapy to a population of patients with a very rare cancer,” said Dr. Elad Sharon, M.D., of CTEP and a study coauthor.
For Dr. Nghiem, the new findings have expanded the treatment options for patients who need them. “As a physician, I have told many patients with this disease that we had nothing more to offer them,” he said. “It’s truly exciting to now be able to give them more options.”