Avasopasem Shields Normal Cells from Radiation, Helps Kill Cancer Cells
, by Sharon Reynolds
Cancer treatments aim to kill cancer cells. Other treatments often used to help people with cancer, called supportive therapies, protect normal tissues or make the side effects from cancer treatments more bearable. What if one drug could play both of these roles at the same time?
In new studies in mice, researchers found that a drug called avasopasem manganese (AVA), which has been found to protect normal tissues from radiation therapy, can also make cancer cells more vulnerable to radiation treatment.
AVA provides this dual effect by exploiting the differences in the way normal and tumor cells produce hydrogen peroxide, explained Douglas Spitz, Ph.D., professor of radiation oncology at the University of Iowa, who helped lead the study.
With any cancer treatment, “you try to find this sweet spot where you’re balanced between an effective therapeutic dose for killing cancer cells, but not causing excessive harm to normal tissues,” said Michael Espey, Ph.D., of NCI’s Division of Cancer Treatment and Diagnosis, who was not involved in the study. “If you can [have a single drug that] lowers the toxicity in normal tissues while increasing the toxicity in cancer cells, then you really have sort of a game changer.”
More work is needed to see if the effects observed in mice can be replicated in people. But in April, Galera Therapeutics, which manufactures AVA, reported positive findings from a small clinical trial of AVA added to a targeted form of radiation therapy in people with pancreatic cancer. Two other ongoing clinical trials are also testing AVA in combination with radiation therapy in lung and pancreatic cancer.
Building on Cells’ Natural Defense Mechanisms
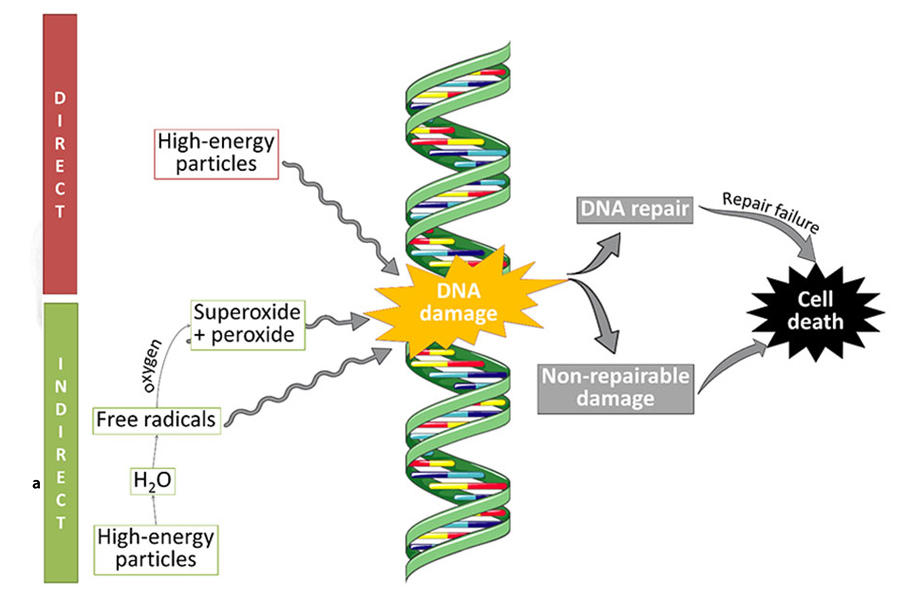
In radiation therapy, high doses of x-rays or other charged particles are aimed at a tumor. The radiation can damage cancer cells’ DNA to the point where the cells stop dividing or die. While a single radiation dose is administered in minutes, many of the resulting changes in cells that cause them to die take days to occur.
When a dose of radiation hits a cell, its high energy creates compounds called free radicals that can damage many parts of the cell, including DNA.
For example, water molecules consist of two hydrogen atoms and one oxygen atom: H2O. A beam of radiation can rip hydrogen atoms from water molecules in a cell. This loss of hydrogen atoms sets off a domino effect, starting with the generation of free radicals that, in turn, fuel the production of hydrogen peroxide, which can damage DNA.
Compounds in cells called free-radical scavengers protect them from normal levels of free radical production and damage. AVA mimics a type of natural free-radical scavenging enzymes called SODs. These enzymes convert a free radical called superoxide into hydrogen peroxide and oxygen. In normal cells, hydrogen peroxide is removed by other enzymes. But in cancer cells, because of their compromised metabolism, the hydrogen peroxide is not removed, causing the formation of additional free radicals that damage DNA.
“It’s a unique concept, that [AVA] mimics something that the body does already,” but has been engineered to do it more efficiently, Dr. Espey said. Accordingly, drugs of this type are called SOD mimetics.
In a recent clinical trial, treatment with AVA was shown to substantially reduce mucositis, sores in the mouth caused by radiation and chemotherapy, in people undergoing treatment for head and neck cancer. The effects of SOD mimetics on cancer cells, however, have not been as closely examined.
“But it was anticipated that [SOD mimetics] would protect normal tissue and sensitize tumor tissue because of the difference in free radical biology between the two,” Dr. Spitz explained. For AVA, the main difference that matters is that cancer cells exposed to radiation process superoxide and other free radicals far less efficiently.
So, while AVA protects normal cells by scavenging the superoxide they produce in response to radiation, it could potentially have the opposite effect in cancer cells. By turning their superoxide into bursts of hydrogen peroxide, AVA treatment could potentially increase the DNA damaging effects of radiation in cancer cells specifically.
A Potent Combination
To test this idea, the two research teams grew human non-small cell lung cancer cells in the legs of mice, then treated them with radiation, with or without AVA. Tumors shrank much more in mice that received AVA just before radiation than in mice that got radiation alone.
When additional doses of AVA were given to the mice daily for 4 days after radiation—during the time cancer cells could be expected to be producing superoxide—the effect was greatly increased, with many tumors disappearing entirely.
“We expected to see an enhanced tumor response, but we never expected the effect to be as large as it was,” said Michael Story, Ph.D., of the University of Texas Southwestern Medical Center, who co-led the study with Dr. Spitz. “And what was more interesting was that the effect was dependent on the size of the radiation dose that we used.”
That is, additional experiments found that AVA enhanced the response to radiation only when that radiation was given at higher doses, such as the levels that would be seen with a more precisely delivered form of radiation called stereotactic body radiation therapy (SBRT), which is commonly used for treating lung cancer. However, the dose of radiation required to totally eliminate tumors was much lower with the addition of AVA than without.
Further experiments in lung cancer cells confirmed that AVA caused a boost in hydrogen peroxide production by lung cancer cells exposed to radiation while the same combination of AVA plus radiation protected normal lung cells.
When the combination of radiation and AVA was given to mice with tumors that produced an abundance of an enzyme that scavenges hydrogen peroxide, there was almost no added benefit, and tumors in those mice continued to grow. These findings, the researchers concluded, indicate that it’s the boost in hydrogen peroxide from AVA that kills the tumor cells after radiation therapy.
Lung cancer cells weren’t the only type of cancer cell that appeared to be extra sensitive to the combination treatment. The researchers also found that AVA enhanced radiation-induced death of both head and neck cancer cells and pancreatic cancer cells.
Based on these results, two clinical trials are underway testing the combination of AVA and radiation therapy in people with lung cancer and pancreatic cancer.
The two trials' goals are slightly different, Dr. Spitz explained. With lung cancer, researchers want to see if adding AVA can protect normal cells enough to use a higher-than-usual dose of radiation—enough to potentially shrink tumors entirely.
Pancreatic cancer is often resistant to treatment with radiation. The new trial in pancreatic cancer is testing whether adding AVA to SBRT can improve how long people with this aggressive cancer live.
Beyond Radiation Therapy
The uses for AVA and similar SOD mimetics may go beyond radiation therapy, Dr. Spitz explained. For example, early studies have suggested that these compounds can protect the kidneys from the damaging effects of the chemotherapy drug cisplatin. In one such study, “everybody who got a full [dose] of cisplatin had chronic kidney disease. But none of those that got AVA did,” he said.
Researchers are also starting to test whether the combination of drugs like AVA and radiation can boost the effectiveness of immune checkpoint inhibitors, a type of immunotherapy, Dr. Story added.
“That’s a very hot topic these days,” said Dr. Espey. The core idea, he explained, is that cancer cell DNA that’s been damaged by radiation can be recognized by parts of the immune system that normally respond to a viral threat. The immune system would then perceive the cancer cells as a target.
“So the idea is that you could prime the immune system using radiation to elicit DNA damage, then [add] immune checkpoint inhibitors,” Dr. Espey explained. The checkpoint inhibitors would then block the mechanisms cancer cells normally use to suppress the immune system and, the hope is, supercharge the immune response.
Researchers have already been testing this idea with radiation alone. But extending the period of hydrogen peroxide production—and the resulting DNA damage—with a compound like AVA has the potential to make the strategy more effective, he added.
Dr. Story is even starting to test whether SOD mimetics could be used to protect astronauts from exposure to ionizing radiation in space.
“I think there’s going to be a bonanza of other uses [for these drugs],” Dr. Spitz said. “There’s going to be other studies in different organs, different diseases, and different toxins. Cancer is the tip of an iceberg.”