PDX Mouse Models Are Reliable Stand-Ins for Human Tumors, Study Finds
, by Carmen Phillips
Mouse models are among the most valuable tools in cancer research. Researchers use them for many types of studies, from identifying possible new cancer treatments to finding new clues about cancer biology.
But mice aren’t humans. So developing mouse models that provide meaningful research results—namely, by ensuring they replicate how cancer behaves in humans as closely as possible—has been a high priority for the research community. Now, a large study from an international group of researchers has provided some strong reassurance that an increasingly relied-upon type of mouse model, known as PDX mice, does just that.
PDX is shorthand for patient-derived xenograft, meaning that the models are initially created by implanting a fragment of a human tumor into a mouse. And this new study shows that the resulting population of these PDX mice largely retain the genetics of the human tumors from which they were initially created. There was also evidence that few substantial changes developed in cancer-related genes in the mouse tumors, the researchers reported January 7 in Nature Genetics.
Coming from the most comprehensive study of its kind done to date, the findings should give researchers confidence about the validity of studies using PDX models, said one of the study’s lead investigators, Jeffrey Chuang, Ph.D., of the Jackson Laboratory for Genomic Medicine.
That’s incredibly important, because results from such studies are increasingly being used to decide whether to move experimental treatments forward into human clinical trials, Dr. Chuang explained. “To get to the clinical trial stage is such a big investment, so you want the best data possible” to make those decisions, he said.
Not surprisingly, the study did show that there is some variation between human tumors and the versions of those tumors that are present in PDX mice, explained Senthil Muthuswamy, Ph.D., of Beth Israel Deaconess Medical Center and Harvard Medical School, who develops research models of cancer but was not involved in this study.
But that’s not surprising, Dr. Muthuswamy continued. “No model is perfect. Just because a model is not a perfect reflection of human tumors, that doesn’t mean it’s not useful,” he continued. “There are previous studies showing that the PDX models have very good utility, and this study conveys that message quite robustly.”
Cancer Research in a “Biological System”
Animal models are essential to some cancer researchers because they allow them to study a tumor in a more biologically relevant manner than is possible with other models, such as cancer cells in a laboratory dish, Dr. Chuang explained.
A tumor in an animal model “is in a growing tissue,” he said. “It has the same characteristics as [human] tumors. There are blood vessels in and around the tumor, there is interaction with” the cells and tissue around the tumor.
For a number of reasons, both biologic and economic, mice are the most commonly used animal model. There are several kinds of mouse models. The most commonly used are those created by injecting mice with cancer cells that have been grown and maintained in laboratory dishes (known as cell lines). That’s because these cells reliably and quickly grow into tumors, providing a relatively inexpensive and easy way to rapidly produce a large number of mice to study.
Although they are an important tool, these models also have important limitations, explained Jeffrey Moscow, M.D., of the Cancer Therapy Evaluation Program in NCI’s Division of Cancer Treatment and Diagnosis, an investigator on the study.
For example, because cancer cell lines are stored and grown in artificial conditions, they tend to undergo numerous genomic changes over time, Dr. Moscow said, meaning they can’t necessarily provide “a faithful representation of a patient’s tumor.”
Making a Better Mouse Model
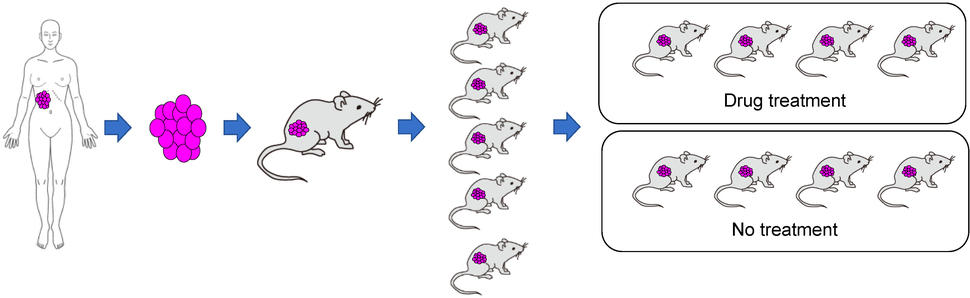
PDX models were developed to help address the shortcomings of other available models. The mice used for PDX models are bred to lack an immune system, which protects the implanted tumor fragment from being attacked and destroyed by the animals’ immune cells.
Once the original human tumor fragment has taken, or engrafted, in a mouse and grows into a full-blown tumor, that original animal is then scaled up. That’s done by taking fragments of its tumor and implanting into several more mice—a process known as passaging.
Once the tumor fragments in those mice have grown into full-blown tumors, further passaging rounds are done until enough of the mice are available to perform a given study. In contrast to models produced with cell lines, creating a PDX model can be a time-consuming process.
“To fully establish a model can take from 6 months to a year,” Dr. Chuang said.
Despite the greater time and cost involved, PDX models have emerged as a research workhorse, particularly for studies involving experimental drugs or drug combinations, Dr. Moscow explained. At NCI, he said, results from PDX model studies “are one of the major criteria we use to prioritize which [drugs] we’re going to move into clinical trials.”
Addressing the Big Question about PDX Models
Recently, questions have been raised about the wisdom of the growing reliance on PDX models.
A chief concern is whether, as the original bit of human tumor travels through multiple rounds of passaging, the evolutionary pressures put on them as they grow in mouse after mouse might cause the tumors to undergo genomic changes, including different changes than those that occur in humans. If there is enough divergence from the original tumor, studies performed in these mice might not reflect what would happen in a human.
A 2017 study from researchers at Harvard and Massachusetts Institute of Technology (MIT) appeared to confirm those fears. The research team found that tumors in PDX mice quickly developed genetic changes known as copy number alterations, in which there are more or less than two copies of particular genes.
The changes arose within a few rounds of passaging, they reported, and differed from the genomic changes seen in the human tumors they analyzed. There was also evidence that the changes seen in the mouse tumors affected how they responded to cancer therapies.
But that study had some important limitations, Dr. Moscow said, in particular that it used an RNA-based method to measure gene expression. This method may not reliably capture changes like copy number alterations in PDX mice, he continued, because the comparisons between the original human tumor and the PDX models can be muddled by the presence of bits of normal human tissue in the original tumor sample.
Meanwhile, several smaller studies reached the opposite conclusion, showing strong parallels between tumors in PDX mice and the human tumors from which they were established.
What was needed was a study to resolve these “contradictory observations,” Dr. Chuang and his colleagues wrote.
A More Comprehensive Analysis of PDX Mice
The new study was conducted by researchers from two large consortia focused on advancing the use and science of PDX models: the NCI Cancer Moonshot–funded PDXNet and its European counterpart, EurOPDX.
To conduct the study, the researchers analyzed samples from more than 500 PDX models and more than 300 matched samples of human tumors. The study covered 16 cancer types and tumors from patients in North America, Europe, and Asia.
In addition to assessing copy number alterations in the PDX mice using the RNA-based technique used in the Harvard/MIT study, this new and larger study went several steps further, employing several DNA-based methods to detect genetic changes, including whole-genome and whole-exome sequencing.
This more comprehensive and collaborative approach allowed the researchers “to more accurately assess genetic changes over time by using many analytic techniques and PDX models from many different laboratories,” Dr. Moscow said.
Providing Confidence on PDX Models
The result was that they did not find extensive copy number alterations between human tumors and those in the PDX models. This was the case even in “late-passage” models—that is, in mice many times removed from the original PDX mouse.
Even when the researchers looked specifically at a trio of matched samples—tumor samples from patients with metastatic colorectal and breast cancer and from their respective early-passage and late-passage models—they largely resembled each other.
Overall, Dr. Chuang said, the study should provide confidence that genomic changes in PDX models “are not a problem.”
Dr. Muthuswamy praised the two groups for conducting the study, stressing that it will help move the science of cancer models forward.
His laboratory develops three-dimensional models called organoids, which also use cancer cells taken directly from patients’ tumors that are induced to grow into “mini-organs” in the laboratory. Unlike cancer cells in a dish, these models can replicate some aspects of how tumors develop in human tissue and maintain the genetic makeup and tissue structure of the original tumors, but they can be developed more rapidly and cheaply than PDX mice.
He believes that organoid models can eventually progress to the point where they can be used as a way to prioritize the most promising drugs to test in PDX models.
Overall, Dr. Muthuswamy said that he’s hopeful that more researchers will begin to use PDX models. Thanks to programs like NCI’s Patient-Derived Models Repository, “the availability of PDX models is much better now,” he said. “These are the next phase of cancer models that we need to use, because they capture some level of patient-to-patient variation. They’re really very important.”