New Way to Classify Meningioma Brain Tumors Suggests Potential Treatments
, by Nadia Jaber
Two separate but complementary studies have found a new way to classify meningioma, a type of tumor that forms in the outer covering of the brain. The new grouping system may help predict whether a patient’s tumor will grow back (recur) after treatment. It also has the potential to reveal what’s causing tumor growth and identify treatment ideas, according to the scientists who led the studies.
The standard way of classifying meningioma is based on the appearance of the tumor cells under a microscope. But the new approach focuses on molecular changes inside the tumor cells. More specifically, both studies looked at chemical marks on DNA—what’s known as DNA methylation. DNA methylation is a type of epigenetic change that fine-tunes gene activity.
The researchers identified several groups of meningiomas with distinct patterns of DNA methylation. These groupings turned out to reflect the chance of tumor recurrence after treatment.
What’s more, tumors in each group had similar biological features, the scientists found. That knowledge, in turn, revealed a few potential treatment options, including cell cycle inhibitors and a drug that targets epigenetic changes.
Both studies converged on similar findings “with some minor variations,” said Kenneth Aldape, M.D., of NCI’s Center for Cancer Research, who, along with Gelareh Zadeh, M.D., Ph.D., of the University of Toronto, led one of the studies. That overlap shows that DNA methylation is a powerful way to classify meningiomas, added Dr. Aldape, whose study was published August 25, 2021, in Nature.
“Despite the fact that [meningioma is] the most common brain tumor, it’s been rather understudied in part because there is a pervasive misconception that meningiomas are benign,” said David Raleigh, M.D., Ph.D., of the University of California, San Francisco, who led the other study, which appeared May 9 in Nature Genetics.
“But that’s not doing justice to all the meningioma patients out there,” he said. Around 20% to 30% of meningiomas are aggressive and potentially deadly.
Current meningioma treatments fall short
For decades, meningiomas have been grouped into tumor grades based on the way the tumor tissue looks under a microscope, including how quickly the tumor cells appear to be growing.
Grade 1 tumors grow slowly, grade 3 tumors grow quickly, and grade 2 tumors are somewhere in between. And although grade 1 tumors grow more slowly, they can push on the brain, causing changes in eyesight, hearing, smell, strength, or other issues.
Doctors use the tumor grade, its location in the brain, and other factors to determine how aggressive the tumor is and to decide what the best treatment is.
While this grading system is useful, it can be imprecise, Dr. Aldape said. Some grade 1 tumors come back after treatment whereas some grade 3 tumors don’t, he explained.
And tumor grading doesn’t give any insight into what’s driving the growth of a patient’s tumor, Dr. Raleigh added. So, a more informative way of classifying meningioma is needed, he said.
New treatment options are also desperately needed, both researchers stressed. Currently, the only options for meningioma are surgery and radiation. But tumors that are close to major nerves or structures can’t be removed by surgery.
And if a tumor grows back or gets worse after surgery and/or radiation, there are no effective treatments. Only half of all people with grade 2 or 3 meningiomas live for more than 10 years after being diagnosed.
Tumor groups that predict recurrence
Both sets of researchers analyzed DNA methylation patterns in tumor samples previously collected from hundreds of people with meningiomas. They then identified groups of tumors with distinct patterns of DNA methylation.
In both studies, the researchers found that these groupings reflected the chance of the tumor growing back after initial treatment. In other words, certain tumor methylation groups had a low chance of tumor recurrence while others had a high chance of recurrence.
The scientists then defined the key biological features of each tumor group by layering in additional data on DNA mutations, RNA levels, and protein levels in the tumor samples.
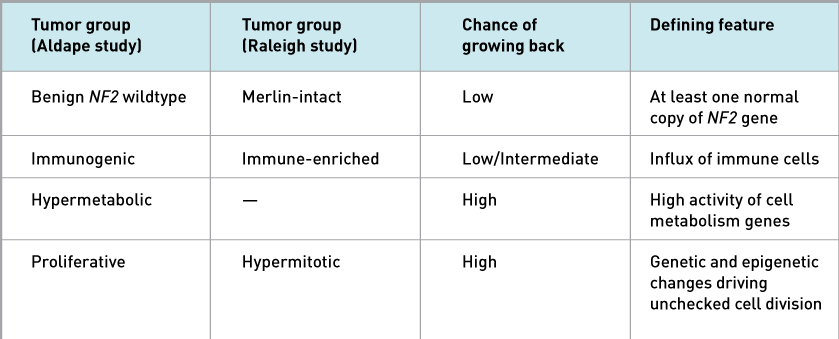
Both studies found that one tumor group (called Benign NF2 wildtype in one study and Merlin-intact in the other) had at least one normal copy of a gene called NF2, which makes a protein called Merlin. Additional cell and mouse experiments by Dr. Raleigh’s group suggest that Merlin prompts meningioma cells to self-destruct upon chemotherapy or radiation treatment.
That may be one reason why people with tumors of this group have a low chance of recurrence, Dr. Raleigh explained.
Another tumor group (Immunogenic/Immune-enriched) was marked by an influx of immune cells, both studies showed. But the immune cells didn’t appear to be killing the tumor cells.
Instead, Dr. Raleigh and his colleagues found that T cells in these tumors were run down, or exhausted. They also saw that Immune-enriched meningiomas had more lymph vessels, structures that ferry immune cells around the body.
Although immunotherapy is being explored as a treatment option for meningioma, these findings suggest that immunotherapy might not work for patients with Immunogenic/Immune-enriched meningioma, Dr. Raleigh noted.
A third tumor group (Hypermitotic/Proliferative) had genetic and epigenetic changes that led to nonstop cell division, both studies found. These tumors had the highest chance of recurrence.
Dr. Aldape and his colleagues also identified a fourth tumor group (Hypermetabolic) that have a high chance of recurrence and have increased activity of genes that control cell metabolism.
Findings lead to new treatment ideas
Both studies uncovered potential treatment approaches for specific groups of meningiomas.
The team led by Drs. Aldape and Zadeh zeroed in on vorinostat (Zolinza), a drug that reverses certain epigenetic alterations and is used to treat lymphoma. In the lab, vorinostat killed human meningioma cells in the proliferative group but not in the other groups. And in mice, the drug slowed the growth of proliferative tumors.
Meanwhile, Dr. Raleigh and his colleagues tested several cell cycle inhibitors—drugs that block cell division and are used to treat breast cancer—in lab studies. These inhibitors killed Immune-enriched and Hypermitotic meningioma cells grown in the lab, in “mini-brain” organoids, and in human tumors implanted into mice, they found.
Given these results, Dr. Raleigh’s team offered abemaciclib (Verzenio), a cell cycle inhibitor, to three people with recurrent Hypermitotic meningiomas who had run out of treatment options.
“The tumors would not leave these patients alone. They kept coming back despite tons of surgery and radiotherapy,” Dr. Raleigh explained.
Abemaciclib shrank the tumors of all three patients. One of the patients was blind in her right eye and had started to experience blurred vision in her left eye as her tumor got worse.
“We started her on abemaciclib and, to our delight, her vision came back in her left eye and her tumor got smaller when nothing had shrunk it for years,” Dr. Raleigh said. They have treated several more patients with abemaciclib, he added.
Dr. Raleigh and his colleagues at the University of California, San Francisco are developing a clinical trial of abemaciclib for people with meningioma. And a handful of clinical trials evaluating cell cycle inhibitors in people with meningioma are already ongoing, he noted.
Sharing new knowledge
Neither research team is wasting any time sharing and applying their findings.
Dr. Aldape and his colleagues have been analyzing DNA methylation profiles for people around the country with hard-to-treat meningioma. “Here at NIH, we're routinely using DNA methylation profiling not just for meningiomas, but for all brain tumors,” he said.
People with a confirmed diagnosis of a rare brain or spine tumor, including meningioma, can participate in the NCI-CONNECT Clinic, which offers tumor DNA methylation testing. Please contact the NCI-CONNECT team.
Meanwhile, Dr. Raleigh’s group has developed a free meningioma classifier app. When a doctor or researcher plugs a patient’s DNA methylation profile into the app, it tells them what methylation group the tumor falls into.
In addition, both studies have created a treasure trove of data for scientists to continue exploring. That was part of the motivation for their study, Dr. Aldape said. Perhaps even more clues about meningioma biology and new treatment ideas are not too far away.