Molecular Burglary: Cancer Cells Hijack Energy from Immune Cells
, by Sharon Reynolds
In theory, the immune system can recognize cancer cells as foreign and destroy them. In practice, this is often difficult, particularly after a tumor has become established in the body.
And even when immune cells, especially certain killer T cells, make it into a tumor, they face a hostile environment. This can include molecules that can disable T cells, low oxygen, and a lack of nutrients for energy. The end result is often a dysfunctional state known as T-cell exhaustion.
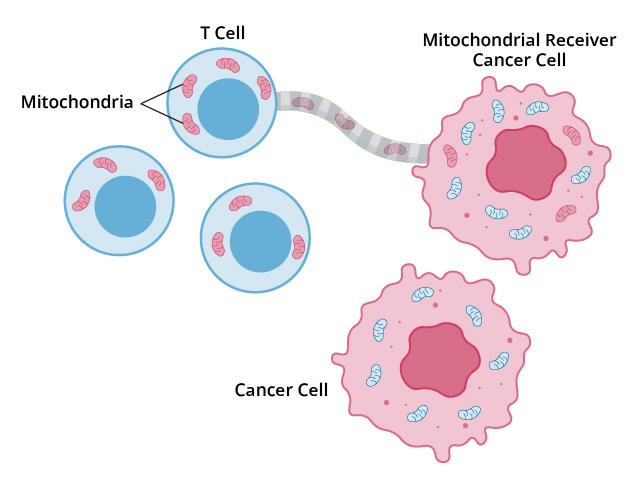
Now, a new study has confirmed the existence of yet another way that tumors can thwart T cells. In some tumors, a subset of cancer cells can act like a thief siphoning fuel from a car’s gas tank: they drain mitochondria—the tiny structures within cells that produce energy—from T cells and use them for their own energy needs.
This act of energy robbery sets up a one-two blow, producing less-efficient T cells and turbo-charged cancer cells, according to the results published on October 9 in Cancer Cell.
However, a silver lining is that any process cancer cells use to survive is a potential vulnerability. It’s not yet fully understood what triggers this mitochondrial hijacking. But once that process is teased apart, “it could serve as a future target [for treatment],” said Bo Li, Ph.D., of the Children’s Hospital of Philadelphia, who led the new study.
Researchers have known for some time that, in the environment in and around tumors, "there was an exchange of nutrients, other molecules, and even organelles for metabolic survival,” said Konstantin Salnikow, Ph.D., of NCI’s Division of Cancer Biology, who was not involved in the study.
“But here we learned that the mitochondria can be drained by cancer cells," Dr. Salnikow continued. "This suggests yet another way that the mechanisms tumor cells use to survive can lead to the dysfunction of nearby T cells. This is really something new.”
Sharing—or stealing—mitochondria to survive
That mitochondria can be swapped between cells is a relatively recent understanding. In the mid-2000s, this phenomenon was thought to be a friendly one, whereby healthy cells in an injured area of tissue could “donate” their mitochondria to other cells to speed the healing process.
But in 2006, researchers looking at cells grown in lab dishes put a more sinister face on mitochondrial transfer. When placed in a stressful, low-oxygen environment, certain cells tended to lose their mitochondria over time. But when subsequently mingled with other, healthy cells, they appeared to steal mitochondria from those cells to survive. How exactly this theft happened remained poorly understood.
Then in 2021, researchers saw this phenomenon happening in real time, with cancer cells. They observed those cancer cells literally sucking the mitochondria out of nearby immune cells in lab dishes with a straw-like structure called a nanotube.
But does this vampiric phenomenon happen in living organisms? There’s currently no way to visually track mitochondrial transfer in people, Dr. Li explained.
“So that motivated us to develop a tool to track this mitochondrial transport process, using genomic sequencing data,” he said.
Mitochondria have their own genome, which is distinct from that found in the nucleus of cells. This makes it easy to sort which genes in a given cell come specifically from the mitochondria.
So Dr. Li and his team built a statistical method they called MERCI, which can analyze genomic data—specifically RNA—from single cells and track the passage of mitochondria, based on their RNA, from cell to cell. This let them identify a particular population of cancer cells they called mitochondrial receivers.
A fingerprint of mitochondrial theft
After confirming that MERCI could precisely pick out these receiver cells, the researchers used it to analyze tumor samples from people with skin and esophageal cancer.
They found that tumor cells identified as mitochondrial receivers by MERCI had increased activity of genes associated with both nanotube formation and energy production—signs that they could produce nanotubes to siphon off mitochondria, then use those mitochondria to produce more energy. Similar patterns of gene expression were seen across receiver cells from the two cancer types.
Next, they expanded their analysis to include tumor samples taken from people with other cancers, including lung, pancreatic, colorectal, and breast cancers. Again, they identified a subset of mitochondrial receiver cells in many tumors that displayed the hallmark gene expression pattern of mitochondrial theft.
The team next winnowed this gene-expression pattern down to a group of 17 specific genes, known as a gene signature, that specifically identified mitochondrial receiver cells. They then tested the signature on more than 10,000 tumor and normal tissue samples, representing 22 distinct types of cancer, collected by The Cancer Genome Atlas. As part of this process, they assigned a “mitochondrial receiver score” for each tumor, based on the activity of each of the 17 genes in the sample.
In most cancer types, tumor samples had much higher mitochondrial receiver scores than samples taken from adjacent normal tissues, they found. Samples with higher mitochondrial receiver scores also showed signs of more aggressive cell division.
In addition, people whose tumors had higher mitochondrial receiver scores tended to not live as long as those with lower scores—a pattern that was repeated across many of the cancer types they studied.
A new vulnerability for cancer cells?
Now that this process has been confirmed to exist, can it be targeted with new treatments?
“We’ve been thinking about this question for the last 2 years,” said Dr. Li.
Although the 17-gene signature accurately identified mitochondrial receiver cells, many of these cells also had other notable genetic changes. Some of these changes are related to the behaviors needed to steal mitochondria and could be potentially shut down with drugs, he explained.
For example, they include genes in cellular communication pathways involved in altering a cell’s internal “skeleton,” which are needed to produce the hijacking nanotubes.
“So we believe a hint for future [treatments] could be among those pathways,” Dr. Li said.
To that end, his team is trying to identify a drug that can stop the nanotubes in these specific cancer cells from elongating enough to attach to T cells. “But it’s a bit challenging, because we found a couple hundred genes that could potentially be involved” in that process, he said.
The team also wants to study the T-cell side of the equation: whether some T cells may be more susceptible to having their mitochondria snatched than others, and if so, why; where in the tumor this process occurs; and how it might be potentially prevented.
And, if T cells’ ability to produce energy has been impaired by the loss of mitochondria, “is there a way to restore that function?” Dr. Li asked.
It is also important to better understand why this phenomenon happens, explained Dr. Salnikow. It may be a survival response to low-oxygen environments, or it could be a direct defense against the immune system by tumor cells, he said.
The new tool developed by Dr. Li and his team will, for the first time, let researchers start to answer these questions, he explained.
“[MERCI] lays the basis for further work to answer all these questions,” Dr. Salnikow said. “Many labs now have single-cell sequencing [capabilities], so many labs now have the opportunity to investigate mitochondrial theft in more detail.”