No Glucose? Pancreatic Cancer May Have a Ready Energy Alternative
, by Carmen Phillips
A new study has revealed some important information about the behavior of one of the most notorious forms of cancer. Pancreatic cancer, the study found, can readily turn to an alternate source of energy to survive when its primary source, the sugar molecule glucose, is in short supply.
Tumors in the pancreas typically develop a dense, nest-like structure around them—an area often referred to as the tumor microenvironment—and they also often lack intact blood vessels. This unruly architecture surrounding these tumors creates conditions that decrease the supply of glucose, an essential fuel source for normal and cancer cells.
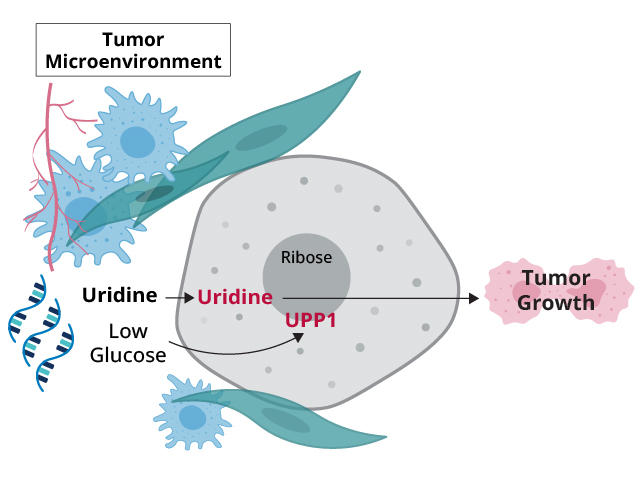
In the study, funded in part by NCI, an international research team showed that pancreatic cancer cells appear to have a potent strategy for overcoming this glucose deprivation: They use an alternative source of fuel, a molecule called uridine.
In experiments involving human pancreatic cancer cells grown in laboratory dishes, they showed that when glucose was lacking, uridine became the main energy source for the cells. And when pancreatic cancer cells that could not use uridine were implanted in mice, only small tumors could form, according to findings published May 17 in Nature.
A related study, published the same day in Nature Metabolism, provided strong confirmation of the finding. In that study, researchers reported that other types of cancer cells could also turn to uridine for energy when they lacked access to glucose.
More research is needed to see if there’s a way to use this information to develop new treatments for pancreatic cancer, acknowledged one of the Nature study’s lead investigators, Costas Lyssiotis, Ph.D., from the University of Michigan Medical School.
Blocking how cancer cells acquire and use energy, or their metabolism, as a treatment has been challenging, Dr. Lyssiotis explained. But a better understanding of how cancer cells adapt their metabolism in the often oxygen- and nutrient-deprived environments in which they exist, he said, may open other avenues for attacking them.
Identifying alternative sources of energy for cancer cells
Pancreatic cancer is one of the leading causes of death from cancer. Not only does its stark microenvironment thwart the entry of drugs designed to kill tumors, but numerous studies have shown that other residents in and around the tumors create an ecosystem that help the tumors thrive.
But this microenvironment also has a downside for tumors: it reduces the amount of oxygen that can flow to them. Pancreatic tumors, in particular, are “highly avascular,” Dr. Lyssiotis said. And not only do they often lack blood vessels, but those that do form are often leaky and mangled.
According to Konstantin Salnikow, Ph.D., of NCI’s Division of Cancer Biology, this abnormal vasculature limits the nutrients available to tumors and can cause “glucose starvation.”
Other studies have shown that pancreatic tumors can get energy from sources other than glucose. But Dr. Lyssiotis’ lab team, along with colleagues from the Institute for Cancer Research, London, wanted to go deeper and identify the specific nutrients that pancreatic tumor cells take in to support their energy needs.
Uridine: Not the typical fuel for cancer cells
The research team began by using an advanced testing platform specifically designed to analyze the nutrients in blood and other tissues. Using the tool, they assessed how readily more than 175 types of nutrients were taken up by about 20 different human pancreatic cancer cell lines (pancreatic cancer cells kept alive in lab dishes) when glucose wasn’t available.
The analysis turned up many of the “usual suspects” that cancer cells would be expected to use for fuel, Dr. Lyssiotis explained. But uridine stood out.
To begin with, uridine is a structural component of RNA, so it’s different than the typical energy sources cells rely on, including carbohydrates like glucose, Dr. Lyssiotis said. It was also readily used by all the pancreatic cancer cell lines they tested.
In addition, they found strong links between high activity of a gene called UPP1 and pancreatic cancer cells’ use of uridine. The latter finding is an important piece of the puzzle, Dr. Lyssiotis explained.
Like a sidecar on a motorcycle, a sugar molecule called ribose is attached to uridine. Ribose is also a component of ATP, the primary energy carrier in cells. UPP1, the protein created when UPP1 is active, interacts with uridine in cells to release ribose, which cells can then use to meet their energy needs.
Analyses of tumor samples from people with pancreatic cancer revealed a link between high levels of UPP1 and shorter survival. And other experiments involving cancer cell lines strongly suggested that UPP1 was stripping uridine of ribose, which the cells then used for energy.
Analyses in mice with pancreatic tumors confirmed what the researchers saw in the cell line experiments. And when they injected mice with pancreatic cancer cells that lacked a functional UPP1 gene—meaning the cells could not produce the UPP1 protein—the resulting tumors were much smaller and more sluggish than those that formed from cells that had an intact form of the gene.
“We were quite surprised by how potent an effect” having no UPP1 had on tumor growth, Dr. Lyssiotis said.
Where are cancer cells getting the uridine? RNA!
Findings from the Nature Metabolism study—funded in party by NCI and conducted by researchers from Harvard, MIT, and the University of Lausanne in Switzerland—largely aligned with those from the Nature study.
The study showed that some cancer cells, including skin and brain cancer, gobbled up uridine to fuel their energy needs when glucose wasn’t available. Other cell types, including immune cells, did as well. The researchers also reported that UPP1 (and a related gene, UPP2) were highly active in cells when glucose was restricted.
But a key question for both research teams was: Where were cells getting the uridine? Dr. Lyssiotis’ team’s analysis pointed to multiple sources in and around the tumor, including immune cells called macrophages. But the companion study pointed directly at RNA.
Indeed, when cancer cells deprived of glucose were given access to plentiful amounts of RNA, they grew just like they would if glucose were present.
“I remember telling my friends that I did a crazy experiment where I tried to feed cells with RNA,” said one of the study’s co-leaders, Alexis Jourdain, Ph.D., of the University of Lausanne, in a news release. “I did not think this was going to work. I was very surprised to see the cells grow.”
Dr. Lyssiotis agreed that RNA appears likely to be a primary source of uridine for cancer cells when glucose in limited.
That cancer cells are getting uridine from RNA “has important implications for cancer biology,” Dr. Salnikow said. “RNA is a very abundant molecule,” he continued, so tumor cells of all types may be relying on it to fuel their metabolism.
It’s also possible that RNA isn’t the sole uridine source for glucose-starved cancer cells, said Michael Pacold, M.D., Ph.D., a radiation oncologist at NYU Langone’s Perlmutter Cancer Center whose research focuses on cancer cell metabolism.
“There are most likely multiple sources,” Dr. Pacold said. When it comes to tumors getting the energy they need, he continued, they are “incredibly adaptable.”
Targeting metabolism as a cancer treatment
With few effective treatments for pancreatic cancer, an obvious question is whether any of these findings suggest possibilities for new therapies, Dr. Lyssiotis said.
Although there’s been tremendous progress in understanding cancer cell metabolism, there is still a lot of work to be done to find ways to exploit it for potential treatment, he explained. Pancreatic cancer, in particular, is challenging.
Pancreatic cancer cells “are professional scavengers,” he said. “If you shut off one fuel source, they will find another.”
Many long-used and highly effective chemotherapy drugs work by interfering with cell metabolism (they are often called antimetabolites), Dr. Pacold said, so there’s already a strong precedent for targeting specific aspects of cancer cell metabolism.
In fact, a host of experimental drugs that disrupt metabolism in different ways are being tested in clinical trials.
Dr. Pacold said he is hopeful that “identifying additional metabolism-related dependencies will be useful in developing improved treatments for cancer.”