Coming Full Circle on Cancer and Extrachromosomal DNA
, by Carmen Phillips
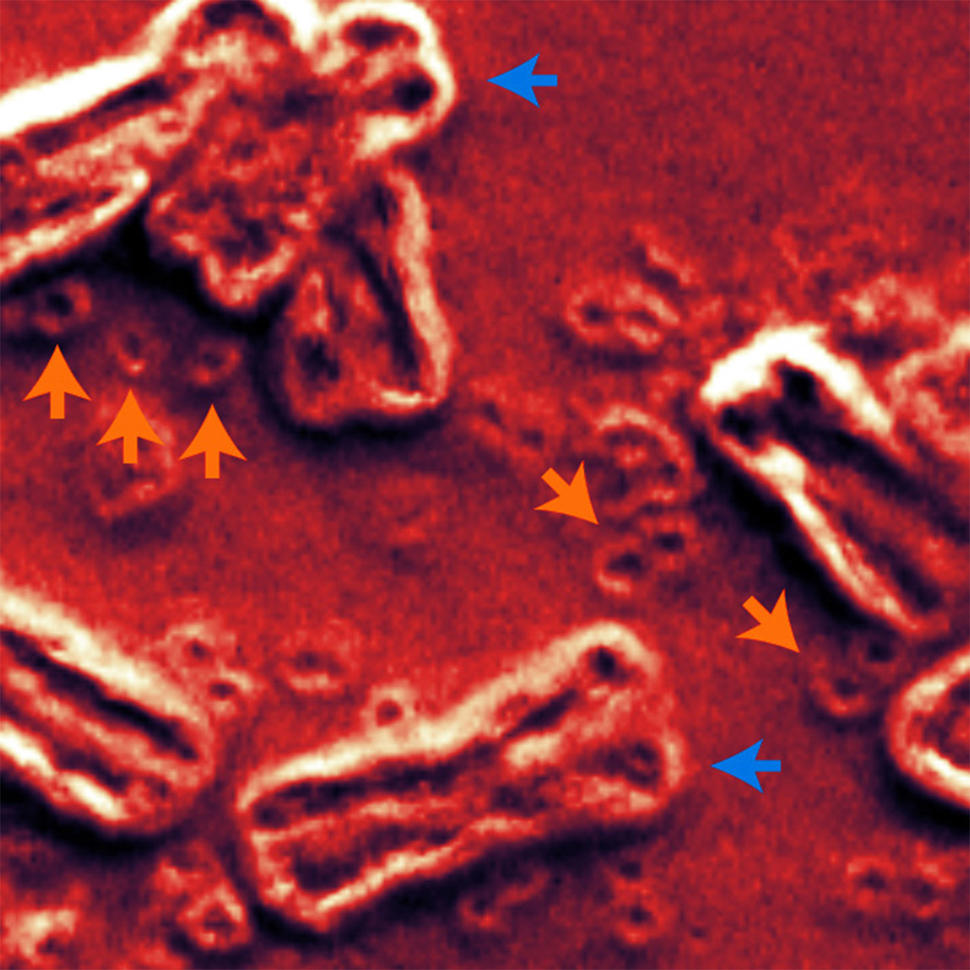
A new study has brought researchers a step closer to understanding the role an unusual form of DNA can play in cancer. This form of DNA—called extrachromosomal DNA (ecDNA)—doesn’t reside on chromosomes, the long tendrils of tightly packaged DNA found inside the nucleus of cells. Instead, circular ecDNA particles float around the nucleus like a child’s lost inner tube in a swimming pool.
A series of recent studies have linked ecDNA to cancer—namely, finding that its presence may help tumors become resistant to treatments and reduce how long people with cancer live. In this new study, researchers wanted to see if ecDNA could be found before cancer had set in. To do so, they focused on esophageal cancer and a condition called Barrett’s esophagus. In a very small percentage of people, Barrett’s esophagus develops into cancer.
The research team’s analyses didn’t prove that ecDNA in Barrett’s esophagus causes esophageal cancer. But they provided the strongest evidence to date that ecDNA directly influences the initial formation and continued progression of esophageal cancer.
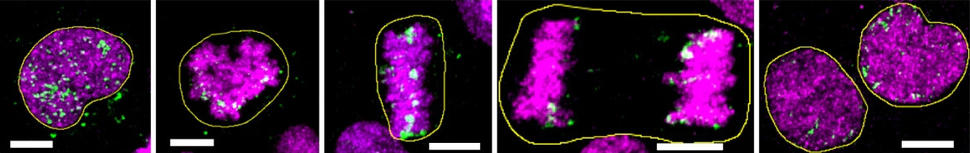
For example, they showed for the first time that ecDNA is indeed present in precancerous tissue and not just cancer cells. And the analyses strongly suggested that when ecDNA is present in precancerous tissue, then that tissue is almost certain to become cancer—at least in the case of Barrett’s esophagus.
Other key findings included that ecDNA volume in tumors increased as they progressed from early to late stages and that the circular DNA often had an array of cancer-causing genes, known as oncogenes.
Published April 13 in Nature, the study was funded through a collaborative initiative between NCI and Cancer Research UK called Cancer Grand Challenges (CGC). The CGC’s goal is to support large research teams that will address some of the most challenging problems in cancer.
“I think [this study] has taught us some important biological lessons,” said one of the study’s lead investigators, Paul Mischel, M.D., of the Stanford University School of Medicine. “The first is that it takes away this idea that ecDNA is a late comer [in the development of cancer]…. No, it can arise early."
Although the study looked specifically at esophageal cancer and Barrett’s esophagus, its findings, along with those from other recent studies of ecDNA, strongly suggest that ecDNA is directly involved in the development of many other cancers, said Ian Fingerman, Ph.D., of NCI’s Division of Cancer Biology.
Although further studies are needed, Dr. Fingerman continued, “There’s no reason to think that may not be the case.”
ecDNA and cancer
The DNA in most human cells is housed on 23 pairs of chromosomes that reside in the nucleus.
In the 1960s, scientists discovered the existence of large snippets of DNA in the nucleus of cancer cells that appeared to have been jettisoned from chromosomes. Interest in this floating DNA among researchers never really took hold, at least until the 2010s, said Dr. Mischel, one of the first researchers to identify ecDNA as a potential contributor to cancer.
During the last 5 to 10 years, however, thanks to technological leaps—including whole-genome sequencing, advanced imaging, and sophisticated computational tools—researchers have been able to unlock some of ecDNA’s secrets. For example, although there were clues that ecDNA is circular, it wasn’t until 2019 that a study led by Dr. Mischel confirmed that it forms a ring shape.
Some of the same studies linking ecDNA’s presence to tumor resistance to certain cancer treatments and shorter survival have also found that they frequently contain oncogenes, and often many copies of those oncogenes (known as amplification).
That’s particularly important, Dr. Mischel explained, because previous studies he helped lead have shown that, by virtue of their round shape, ecDNA can more rapidly ramp up the activity of these cancer-causing genes and to a greater degree than chromosome-bound genes.
He also stressed another important point about ecDNA. Because ecDNA aren’t attached to chromosomes, they aren’t evenly distributed during cell division like chromosomes and their DNA cargo. Rather, they are randomly parceled to cancer cells as those cells divide and grow in number. As a result, “tumors can reach very high levels of cancer-causing genes… and achieve high levels of genetic variability,” he said.
But even with these findings, the extent of ecDNA’s direct involvement in cancer has been unclear. According to Dr. Fingerman, one big question has been whether they are a root cause of cancer or if they only emerge once “genomic instability” has set in, cell division has gone totally awry, and tumors have formed.
From Cancer Grand Challenges to Barrett’s esophagus and esophageal cancer
Dr. Mischel leads an international team of researchers who were one of the first four groups to receive CGC funding in 2022. The CGC initiative “put in place the kind of framework that allows for this kind of ambitious team science,” Dr. Mischel said.
With their sizable funding award came the task of helping answer the key unresolved questions around ecDNA. Barrett’s esophagus and esophageal cancer offered an ideal opportunity to do just that, he continued.
Although Barrett’s esophagus is a precancerous condition, brought on by long-standing gastroesophageal reflux, it only progresses to esophageal cancer in about 1% of the people who have it. It also appears to progress in distinct stages, with the tissue in the esophagus undergoing abnormal physical changes that trace a path: from low-grade dysplasia to high-grade dysplasia to full-blown cancer.
For this study, the CGC team joined with colleagues from Cambridge University Hospital in the United Kingdom and Fred Hutchinson Cancer Center in Seattle who, as part of long-term studies, had a valuable trove of tissue samples from people with Barrett’s esophagus and esophageal cancer. The Fred Hutchinson team, in fact, had multiple samples that had been collected over several years or longer from people whose Barrett’s developed into cancer and in whom that progression hadn’t occurred.
This latter bunch of samples, Dr. Mischel explained, provided a unique chance to learn from patients by performing a before-and-after examination—a pre- and post-cancer/no cancer time window—in which they could home in specifically on ecDNA.
First indications that ecDNA can fuel cancer
The Cambridge group had 200 samples from people with Barrett’s esophagus and esophageal cancer. Whereas the Fred Hutchinson group provided samples from 40 people with Barrett’s that progressed to cancer and from 40 in which it didn’t.
When the researchers looked across samples from both groups, no ecDNA was found in any from people with Barrett’s esophagus that had low-grade dysplasia. But in Barrett’s esophagus with high-grade dysplasia, it was a different story.
In the Fred Hutchinson samples, for example, every person with high-grade dysplasia that had ecDNA went on to develop cancer. By contrast, among the people whose Barrett’s esophagus with high-grade dysplasia that did not go on to become cancer, only one had ecDNA, and in only a single sample. That person died from another cause.
Further, the frequency of ecDNA (and its level of oncogene amplification) increased as tumors progressed from early-stage to late-stage cancers. In the Cambridge samples, for example, 25% of early-stage esophageal cancer samples had ecDNA, compared with 43% of late-stage samples. In addition, the amount of ecDNA in cells was much higher in the late-stage esophageal cancer samples than in the early-stage samples.
Another key finding came from the small group of samples from people who went on to develop cancer: a strong link between the presence of ecDNA and alterations in a gene known as TP53.
Often referred to as the “Guardian of the Genome,” TP53 is one of the most important tumor suppressor genes. It directs activity that can repair damaged DNA in healthy cells or, if DNA damage is too extensive and uncontrolled cell growth is likely, push cells toward death. Alterations to TP53, however, can prevent it from performing these duties.
Changes in TP53 were present in all eight of the people who went on to develop cancer and in whom ecDNA were found prior to their cancer diagnosis, they reported.
The consistent finding of TP53 alterations in this group, Dr. Mischel said, suggests that this change “is critical to ‘setting the soil’ in which ecDNA can arise.”
Further analyses, other cancers
The research team is performing new analyses to follow-up on several potentially important clues in the existing data, Dr. Mischel said. Those analyses include looking more closely at the events that led to ecDNA formation and at the activity of other cancer-related genes in ecDNA. They’re also developing similar studies for other cancers.
As for esophageal cancer, the study’s findings are unlikely to have an immediate impact, wrote David Wang, M.D., who specializes in treating this cancer at the University of Texas Southwestern Medical Center, in an accompanying editorial in Nature.
Dr. Wang agreed that the study’s findings point to an important role for ecDNA in the progression from Barrett's esophagus to esophageal cancer. But, he noted, further studies are needed to determine how to apply what’s known so far about ecDNA to better combat the disease.
“Future work,” he wrote, “should focus on therapeutic interventions relating to [ecDNA].”
While treatment improvements are a clear possibility as researchers learn more about ecDNA, Dr. Mischel agreed, this work also may present other clinical opportunities.
“We … need to think about the potential [of using ecDNA] for [cancer] early detection and surveillance strategies,” Dr. Mischel said. “There [are] real opportunities here to begin looking at these things.”