FDA Approves Cobimetinib as Part of Drug Combination for Advanced Melanoma
, by NCI Staff
On November 10, the Food and Drug Administration (FDA) approved the use of cobimetinib (Cotellic™) in combination with vemurafenib (Zelboraf®) to treat patients with metastatic melanoma.
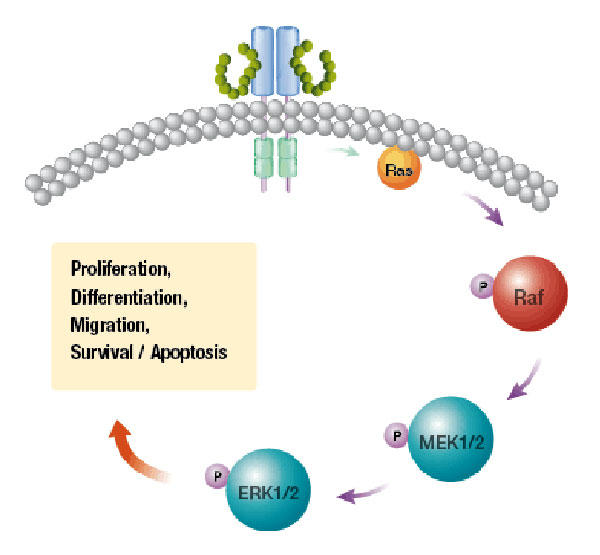
Both drugs block targets that act at different steps in the same signaling pathway, the mitogen-activated protein kinase, or MAPK, pathway. Cobimetinib inhibits the activity of an enzyme known as MEK, and vemurafenib inhibits the enzyme BRAF.
The approval was based on the effectiveness of cobimetinib plus vemurafenib in a randomized phase III clinical trial of 495 patients whose tumors had specific mutations in the BRAF gene and who were not candidates for surgery.
Patients enrolled in the clinical trial received vemurafenib—which is already approved as a single agent for patients with advanced melanoma whose tumors have BRAF mutations—in combination with either cobimetinib or a placebo.
Patients who received vemurafenib plus cobimetinib had a median progression-free survival of 12.3 months, compared with 7.2 month in patients who received vemurafenib plus placebo.
At 17 months after initiating treatment, about 65 percent of patients who received the two-drug combination were still alive, compared with 50 percent of those who received vemurafenib alone.
The most common side effects in patients who receive both drugs include diarrhea, photosensitivity, and vomiting. Cobimetinib may also cause severe side effects, including damage to the heart muscle or to other muscles, new skin tumors (primary cutaneous malignancies), retinal detachment, severe skin rash, and liver damage.
The improvement in progression-free survival suggests that tumors take longer to become resistant to both drugs given together than they do to vemurafenib alone, explained Howard Streicher, M.D., of NCI’s Cancer Therapy Evaluation Program.
With this new approval, there are now two FDA-approved cancer treatments that combine a BRAF inhibitor with a MEK inhibitor. In 2014, the FDA approved dabrafenib (Tafinlar®) plus trametinib (Mekinist®) as a first-line therapy for patients with metastatic melanoma that has BRAF mutations.
The availability of so many new therapies for melanoma has “raised expectations for patients that malignant cutaneous melanoma can be treated and controlled for many years,” said Dr. Streicher.
With several immunotherapy drugs now approved for melanoma, “an important question is how these combinations will be used alongside the immune-based treatments,” he said. “Both combinations may be used either before or after checkpoint inhibitors.” An NCI-sponsored phase III trial to test which sequence is most effective is currently accruing patients, Dr. Streicher added.