For Rare Melanoma, Checkpoint Inhibitors May Hold Substantial Promise
, by NCI Staff
A new study suggests that patients with a rare form of melanoma, called desmoplastic melanoma, may be particularly likely to benefit from treatments that work by enlisting the immune system to attack tumors.
Immunotherapy drugs called immune checkpoint inhibitors have proven to be very effective in patients with melanoma. Findings from this new study suggest, however, that patients with desmoplastic melanoma may be even more likely to have their tumors shrink after treatment with checkpoint inhibitors than patients with the more common form of melanoma.
In the study, a research team led by investigators from the UCLA Jonsson Comprehensive Cancer Center analyzed data from a small group of patients with advanced desmoplastic melanoma who had been treated with checkpoint inhibitors. Tumors shrank in 70% of these patients and the cancer was no longer detectable in approximately one-third of those patients, they reported January 18 in Nature.
An ongoing NCI-supported clinical trial is testing the checkpoint inhibitor pembrolizumab (Keytruda®) in patients with desmoplastic melanoma. The trial includes a broader spectrum of patients than those included in the Nature study, including patients with earlier-stage disease, in the hope that the treatment might spare some patients from having to undergo what can be highly disfiguring surgery to remove their tumors.
Patients with desmoplastic melanoma historically have “very limited treatment options,” said Antoni Ribas, M.D., Ph.D., the senior investigator on the Nature study. “The NCI-supported trial gives us a great opportunity to confirm this retrospective observation and potentially improve outcomes of patients who have resectable disease.”
Lots of Mutations: A Therapeutic Opportunity?
Three checkpoint inhibitors have been approved by the Food and Drug Administration (FDA) to treat melanoma. The approvals were based on large clinical trials showing that the drugs can shrink tumors in many patients and, in a smaller percentage of patients, keep the disease from returning for extended periods.
Several factors make melanoma particularly amenable to therapies that work by revving up the immune system. One of the most important factors appears to be that melanoma cells tend to have a large number of genetic mutations.
Multiple studies have shown that tumors with this “high mutational burden” tend to be particularly susceptible to immunotherapies, largely because the genetic mutations cause the tumor cells to produce abnormal proteins, which the immune system identifies as “foreign.”
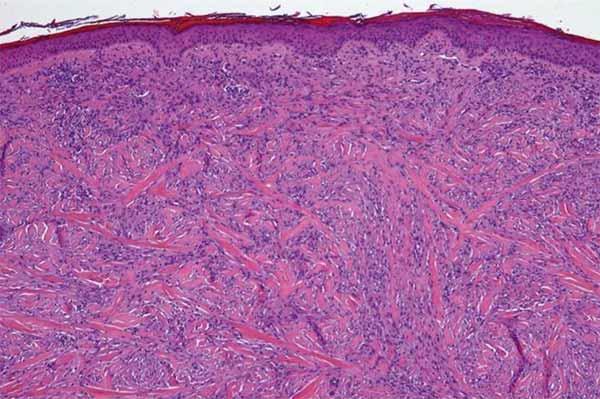
Desmoplastic melanoma accounts for about 4% of melanoma diagnoses. It results primarily from damage to the skin caused by excessive exposure to DNA-damaging ultraviolet light (which is why it forms most often on the head and shoulders).
Desmoplastic melanoma tumors are typically surrounded by thick layers of fibrous tissue and snake along and around nerves. Historically, surgeons “have to do multiple resections to get clean margins” around the tumors, explained Siwen Hu-Lieskovan, M.D., Ph.D., a lead investigator on the Nature study. “Or they may do large resections, hoping to get all of the cancer out.”
That’s why the surgery can be so disfiguring, she continued, often leaving “huge scars on the head and neck regions.”
Studies have shown that tumor cells in patients with desmoplastic melanoma typically have a very large number of genetic mutations.
With that fact in mind, the UCLA-led team gathered data from patients with desmoplastic melanoma who were treated with checkpoint inhibitors to see if that high mutational burden translated into good outcomes.
Rare Cancer, Many Responses
For the study, Dr. Ribas and his colleagues reviewed data from more than 1,000 patients with advanced melanoma who were treated at 10 high-volume cancer centers between 2011 and 2016. All of the patients had been treated with immune checkpoint inhibitors that target the proteins PD-1 or PD-L1.
From that review, they identified 60 patients with desmoplastic melanoma, none of whom had undergone surgery because their disease was too widespread. More than half of these patients had disease characteristics that are associated with a particularly poor prognosis, the research team reported. In the majority of patients in the study, their cancer had gotten worse after being treated with the checkpoint inhibitor ipilimumab (Yervoy®), which targets a protein called CTLA-4.
In patients whose tumors disappeared entirely (a complete response) after treatment with PD-1/PD-L1–targeted checkpoint inhibitors, the response endured and, with a median follow-up of nearly 2 years, none of these patients has experienced a relapse, they reported.
Several patients who experienced only some reduction in the size of their tumors (a partial response) were eventually able to undergo surgery to have them removed. Two of these patients were still alive 5 years later, and one was alive nearly 2 years later, without any recurrence of their cancer.
Tumor cells in many patients had high levels of PD-L1 expression, and there was a large population of T cells in the tumors, “suggesting very active immune responses in these tumors,” said Dr. Hu-Lieskovan.
Because the study is retrospective—that is, it looks back in time at a group of patients—it’s hard to draw firm conclusions from it, Dr. Hu-Lieskovan acknowledged. Nevertheless, she added, the findings still offer a particularly promising outlook.
“This is one of highest response rates to anti-PD-1/PD-L1 immunotherapy that we’ve seen, and it happens to be in a tumor type with one of the highest mutational burdens,” she said.
Clinical Trial: Confirming the Promise
The NCI-funded clinical trial is enrolling patients with advanced and earlier-stage desmoplastic melanoma.
Under FDA’s current approvals for pembrolizumab, patients with any form of advanced melanoma can already receive this treatment. So, for patients with advanced desmoplastic melanoma, the trial will do two things, explained Elad Sharon, M.D., M.P.H., of NCI’s Cancer Therapy Evaluation Program.
First, it will help to confirm the findings from the UCLA team’s retrospective study. And, second, based on studies built into the trial, it could provide other important insights.
Those insights could include more information on how tumor cells change to become resistant to treatment, explained the trial’s principal investigator, Kari Kendra, M.D., Ph.D., of the Ohio State University Comprehensive Cancer Center.
“It can also provide insights into [potential] predictive markers and better guide us in the future with how best to use these therapies,” Dr. Kendra said.
Less Surgery, Better Outcomes?
Patients enrolled in the trial with earlier-stage disease are candidates for, but have yet to undergo, surgery. These patients, Dr. Sharon said, are receiving pembrolizumab prior to surgery (neoadjuvant therapy).
The drug is not approved for use prior to surgery in these patients, he explained.
“Given the high response rate [in patients with advanced disease], we wouldn’t expect the response to be lower for patients with earlier-stage disease,” Dr. Sharon said. “It makes sense to see if we can give it earlier and get a high rate of complete responses.”
Complete responses, or even very good partial responses, could spare many patients from having to undergo surgery at all or reduce the extent of their surgery, Dr. Kendra explained.
Importantly, she added, patients in the trial who are receiving pembrolizumab prior to surgery are undergoing only three cycles of treatment, potentially sparing them from additional side effects.