Drug Combination Improves Outlook for Some Patients with Chronic Lymphocytic Leukemia
, by NCI Staff
For patients with chronic lymphocytic leukemia (CLL) that has stopped responding to treatment, a new drug combination may be an effective option. Based on interim results from a phase 3 clinical trial, investigators reported that, compared with conventional chemotherapy, the combination of venetoclax (VenclextaTM)TM and rituximab (Rituxan®) reduced the risk of cancer progression by more than 80%.
Both drugs are already used to treat CLL, the most common type of leukemia in adults in the United States. But the trial is the first to compare the combination of the two against a conventional treatment regimen for relapsed and refractory CLL, rituximab plus bendamustine (Treanda®).
There was a “profound and very clear difference” in progression-free survival between the two treatment groups, said the lead investigator John Seymour, M.B.B.S., Ph.D., of the Royal Melbourne Hospital in Melbourne, Australia. Dr. Seymour reported the findings at the American Society of Hematology annual meeting on December 12.
“There are a number of drugs that are changing the landscape of CLL, and venetoclax is certainly one of them,” noted Robert Brodsky, M.D., director of the Division of Hematology at Johns Hopkins University, at a press briefing on the trial.
Different Treatments for CLL
Several different treatment options are available for patients with newly diagnosed CLL. Many patients are treated with a combination of chemotherapy and immunotherapy, such as bendamustine plus rituximab, or with a targeted therapy such as ibrutinib (Imbruvica®). But over time, most patients stop responding to the treatment and their cancer returns.
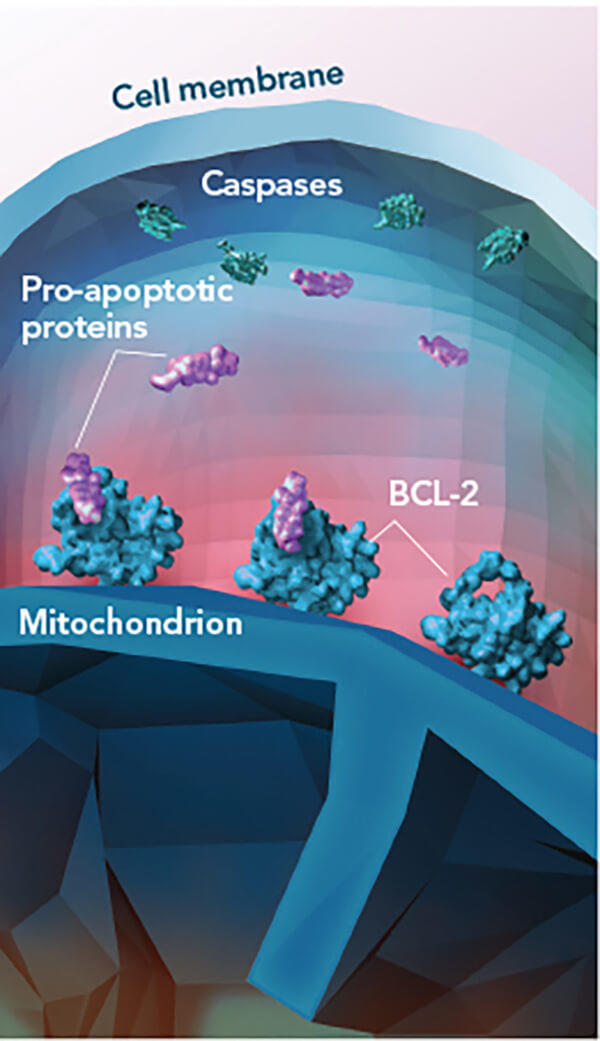
Venetoclax is a targeted therapy that blocks a protein (called BCL-2) that keeps cancer cells alive. It is approved by the Food and Drug Administration (FDA) to treat patients with relapsed or refractory CLL that has a genomic alteration known as deletion 17p. Patients whose leukemia cells have this abnormality do not respond well to conventional chemotherapy.
However, “there are other studies showing that relapsed or refractory CLL responds to venetoclax regardless of [a patient’s] deletion 17p status,” noted Inhye Ahn, M.D., a clinician at NIH’s National Heart, Lung, and Blood Institute.
Researchers “are trying to expand the use of these newly approved agents [because] they are very effective, and they are less toxic” than chemotherapy, she added.
In lab studies, Dr. Seymour and his colleagues found that combining venetoclax with rituximab prevented leukemia growth in mice better than either drug alone.
The MURANO Trial
Based on promising results from early clinical trials of rituximab plus venetoclax in patients with relapsed or refractory CLL, the investigators launched a phase 3 trial called MURANO.
Nearly 400 patients with relapsed or refractory CLL were randomly assigned to receive venetoclax plus rituximab or standard therapy (bendamustine plus rituximab). The trial was sponsored by the two pharmaceutical companies jointly developing venetoclax, AbbVie and Roche.
Because venetoclax kills tumor cells rapidly, it can cause a serious side effect called tumor lysis syndrome. To reduce the risk of tumor lysis syndrome, patients in the venetoclax group were given a gradually increasing dose of the drug over 4 or 5 weeks. They then began receiving rituximab in the 6th week while continuing to receive venetoclax.
At the planned interim analysis, the median progression-free survival was 17 months for patients treated with chemotherapy and was not yet reached for patients treated with venetoclax and rituximab. Approximately 85% of patients in the venetoclax group did not have cancer progression after 2 years of treatment, compared with 36% for patients in the chemotherapy group.
About 27% of patients in both treatment groups had the deletion 17p abnormality. When the investigators analyzed patient outcomes by deletion 17p status, the venetoclax–rituximab combination improved progression-free survival in patients with and without deletion 17p.
More than 25% of patients in the venetoclax group achieved a complete response, compared with 8% in the standard therapy group. More patients in the venetoclax group also had little to no detectable cancer cells in their blood (minimal residual disease–negative).
The venetoclax–rituximab combination also improved overall survival compared with chemotherapy, though a longer follow-up period is needed to see if the benefit persists over time, Dr. Seymour explained.
The investigators did not observe any new safety concerns with the venetoclax–rituximab combination treatment. As had been observed in earlier clinical trials, more patients in the venetoclax group than the chemotherapy group developed high-grade neutropenia, although neither febrile neutropenia nor high-grade infections were more frequent in the venetoclax group.
Considering Study Implications
One aspect of the trial design worth noting, said Dr. Ahn, is the difference in treatment duration between the two groups. Patients in the chemotherapy group received bendamustine plus rituximab for up to 6 months, whereas patients in the venetoclax group received rituximab for 6 months and daily venetoclax for up to 2 years.
Another detail to consider is that “the bendamustine plus rituximab regimen doesn’t work well in patients with deletion 17p,” Dr. Ahn said. Since 27% of patients in the chemotherapy group had deletion 17p, this could have decreased the measures of treatment efficacy.
Nevertheless, she said, the interim trial results “demonstrate that the combination of venetoclax and rituximab is an effective treatment regimen for relapsed or refractory CLL.”
The findings “expand and bring better treatment options to our patients,” said Dr. Brodsky.
Other newly available treatments for patients with CLL include ibrutinib, which is approved by FDA for the treatment of CLL regardless of prior treatment status or genetic changes. And the options may continue to change. One ongoing clinical trial, for example, is testing ibrutinib plus venetoclax in patients with relapsed or refractory CLL.
Dr. Seymour noted that carefully controlled clinical trials are needed to determine which treatment options are the most effective for specific groups of patients.