Sacituzumab Govitecan Earns Full Approval for Triple-Negative Breast Cancer
, by Nadia Jaber
On April 7, the Food and Drug Administration (FDA) granted regular approval to sacituzumab govitecan (Trodelvy) for some patients with triple-negative breast cancer (TNBC). The agency’s action follows last year’s accelerated approval of the drug.
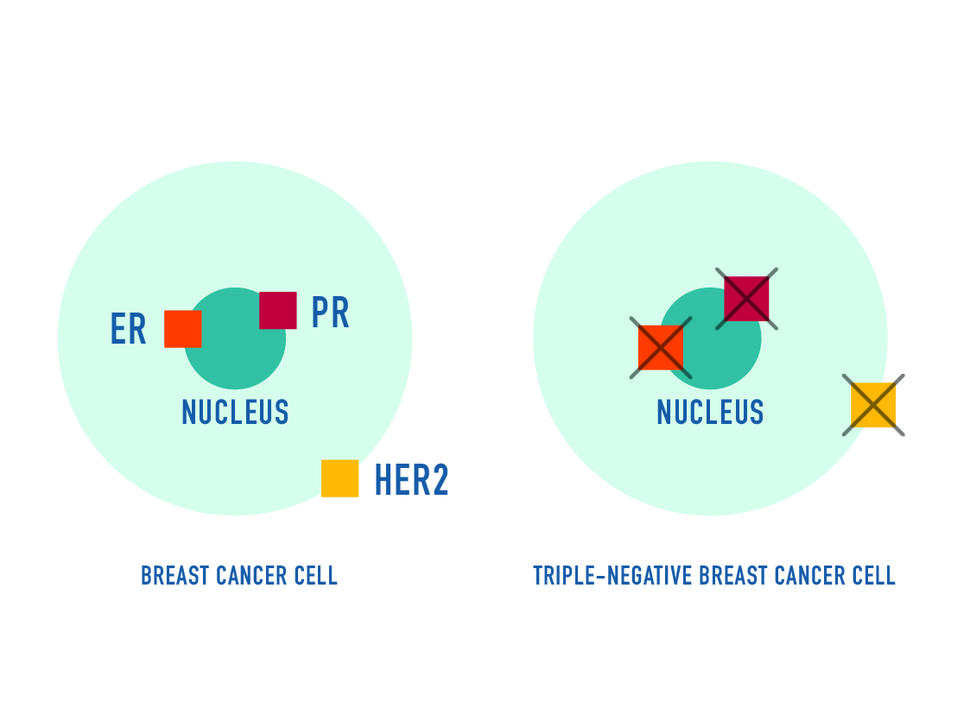
The treatment is approved for people with triple-negative breast cancer that is locally advanced or has spread to other parts of the body and can’t be removed by surgery. Patients must have received two or more breast cancer therapies, including one for metastatic disease, before being treated with sacituzumab.
Promising results from a small study led to the accelerated approval in April 2020. FDA has now upgraded to a regular approval based on findings from a much larger confirmatory clinical trial that showed the drug improved how long patients lived compared with standard chemotherapy treatments.
“This approval validates sacituzumab as an effective new treatment for patients with triple-negative breast cancer,” said Jennifer Matro, M.D., a breast cancer doctor at University of California San Diego Health.
It “provides a much-needed option for patients who have not responded to other therapies,” she added.
NCI’s SBIR: Getting Sacituzumab from the Lab to the Patient
Sacituzumab was initially developed by a small biotechnology company called Immunomedics. NCI’s Small Business Innovation Research (SBIR) program, which supports small businesses that are advancing innovative technologies, provided funding to Immunomedics to help the company launch the first human clinical trials of the drug. Based on positive results in these initial trials, sacituzumab was tested in larger trials, leading to its FDA approval. Immunomedics was acquired by Gilead Sciences in September 2020.
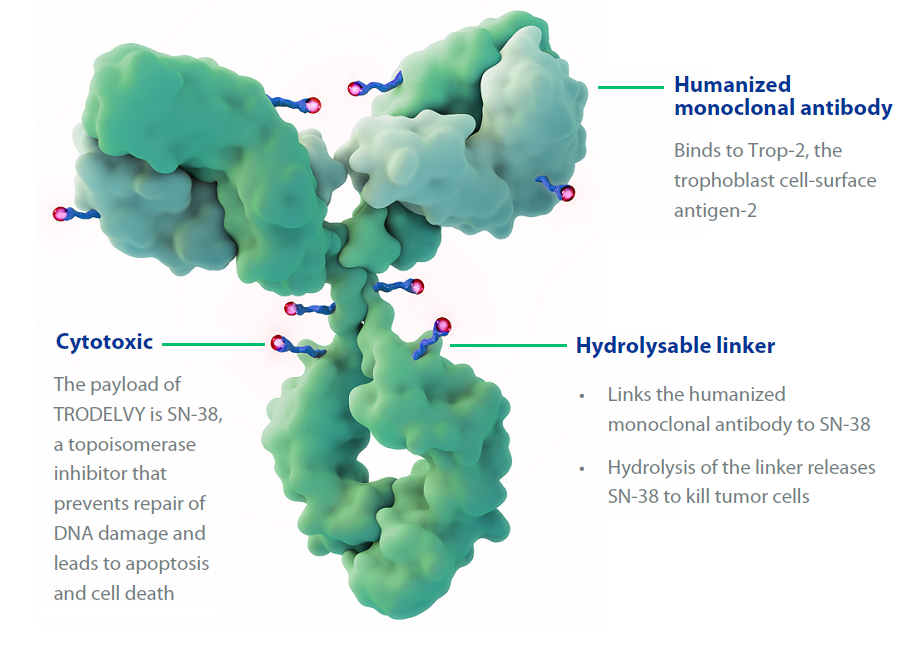
Sacituzumab consists of an antibody coupled to a more potent form of the chemotherapy drug irinotecan (Camptosar). The antibody binds to breast cancer cells, delivering the chemotherapy directly to those cells.
“Historically, the only treatments available for triple-negative breast cancer have been chemotherapies, which are not specifically targeted to the cancer and tend to have more side effects,” Dr. Matro explained.
More than 500 people, all of whom had triple-negative breast cancer that had come back after at least two different chemotherapy treatments, participated in the trial. Among the study participants, 79% were White and 12% were Black.
Twelve percent of patients in the trial had breast cancer that had spread to the brain (what’s known as brain metastases). Nearly half of patients with metastatic triple-negative breast cancer develop brain metastases, which can limit their physical and cognitive abilities, Dr. Matro explained. People with brain metastases often don’t live as long as people without them.
All participants were randomly assigned to receive either sacituzumab or chemotherapy (one of four specific drugs, as selected by their doctor). Sacituzumab is given as an infusion through a vein in the arm.
Measure |
Sacituzumab group |
Chemotherapy group |
|
Months lived without cancer progression (median progression-free survival) |
4.8 |
1.7 |
|
Months lived overall (median overall survival) |
11.8 |
6.9 |
Among all participants, those who received sacituzumab lived a median of 3 months longer without their disease progressing than those who received chemotherapy. And patients in the sacituzumab group lived a median of nearly 5 months longer than those in the chemotherapy group.
“Effective treatments for patients with brain metastases are desperately needed, and to have a clinical trial not only include patients with brain metastases but also demonstrate improvements in disease progression and survival is an important breakthrough for these patients,” Dr. Matro noted.
Among participants without brain metastases, sacituzumab measurably shrank tumors in 35% of those who received it, according to trial results published April 22 in the New England Journal of Medicine. In comparison, chemotherapy measurably shrank tumors in only 5% of patients without brain metastases. For patients without brain metastases, sacituzumab was more effective than chemotherapy regardless of a patient’s age, race, and number or type of previous therapies received.
The most common side effects of sacituzumab were low white blood cell counts (neutropenia), diarrhea, and nausea. In both the sacituzumab and chemotherapy groups, 5% of patients stopped the treatment because of side effects.
The FDA approval comes with a warning for severe or life-threatening neutropenia and severe diarrhea. The agency recommends periodically monitoring patients for these side effects during treatment. Both neutropenia and diarrhea can be treated with additional medicines.
It’s important for patients and their doctors to discuss where sacituzumab may fit into their treatment plan, Dr. Matro noted, particularly as additional data emerge for immunotherapies in select patients with triple-negative breast cancer.