Genetic Signature May Help Tailor Treatment for Meningioma
, by Sharon Reynolds
You can’t judge a book by its cover. And, more and more, scientists are coming to understand that you can’t judge a tumor solely by how its cells look under a microscope.
For meningioma, the most common type of primary brain tumor in adults, researchers have now shown that the activity of a set of 34 specific genes better predicts the clinical behavior of tumors than the physical features of cells in those tumors.
This gene expression signature, the research team found, supplied more accurate information about how aggressive a meningioma is, as well as how likely it is to respond to and come back after treatment, they reported November 9 in Nature Medicine.
In one analysis of more than 100 people who had undergone surgery to have their tumors removed, the researchers showed that using the gene expression signature to evaluate the risk of cancer returning potentially could have spared nearly one-third from also having radiation therapy.
Conversely, a smaller number of other patients who did not undergo radiation after surgery likely should have, because the score revealed that their tumors were more aggressive than initially thought.
“Once these tumors come back [after surgery], they become progressively harder to treat. So, if there’s going to be a benefit to radiotherapy, we’d like to offer that treatment as early as possible,” explained David Raleigh, M.D., Ph.D., of the University of California, San Francisco (UCSF), who led the study.
For meningiomas, basing treatment on cellular appearance alone “has been vexing,” said Mark Gilbert, M.D., of NCI’s Neuro-Oncology Branch, who was not involved in the study. That’s because although standard microscopic analysis can help identify whether patients may be at higher risk of their cancer coming back, its overall accuracy is limited.
“With the [current] classification system, too often there are tumors that don't behave the way they're supposed to," Dr. Gilbert said.
The 34-gene signature’s accuracy needs to be confirmed in additional studies, he added, but it could help refine treatment for many people with meningioma, which could increase how long they live and their quality of life.
A pressing need for better meningioma classification
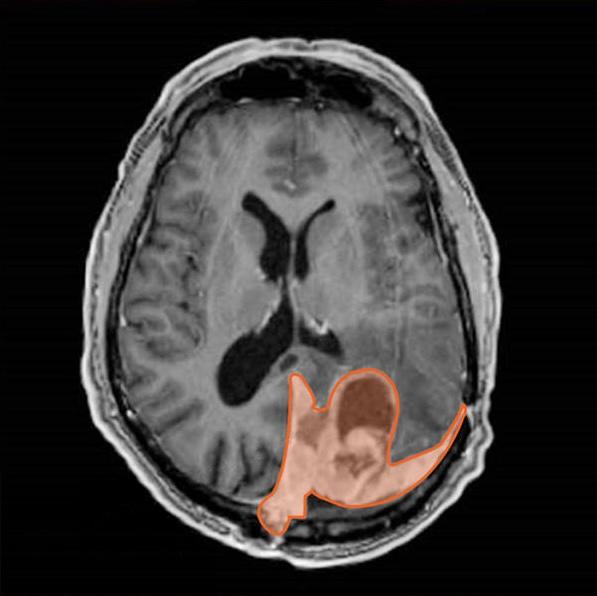
Meningiomas arise from the meninges, the thin layer of tissue that covers and protects the brain and spinal cord. They tend to grow very slowly, sometimes taking years to grow by just a few centimeters. As a result, they’re often referred to as benign tumors.
“But that’s not a term I like to use for anything growing next to the brain,” which is sensitive to even the slightest pressure on its delicate structures that affect essential functions, said Dr. Raleigh. In addition, a subset of meningiomas can grow quickly and invade surrounding brain or bone, making them difficult to control with surgery alone.
So how can doctors sort the slower growing meningiomas from those that will grow quickly or may return rapidly after treatment?
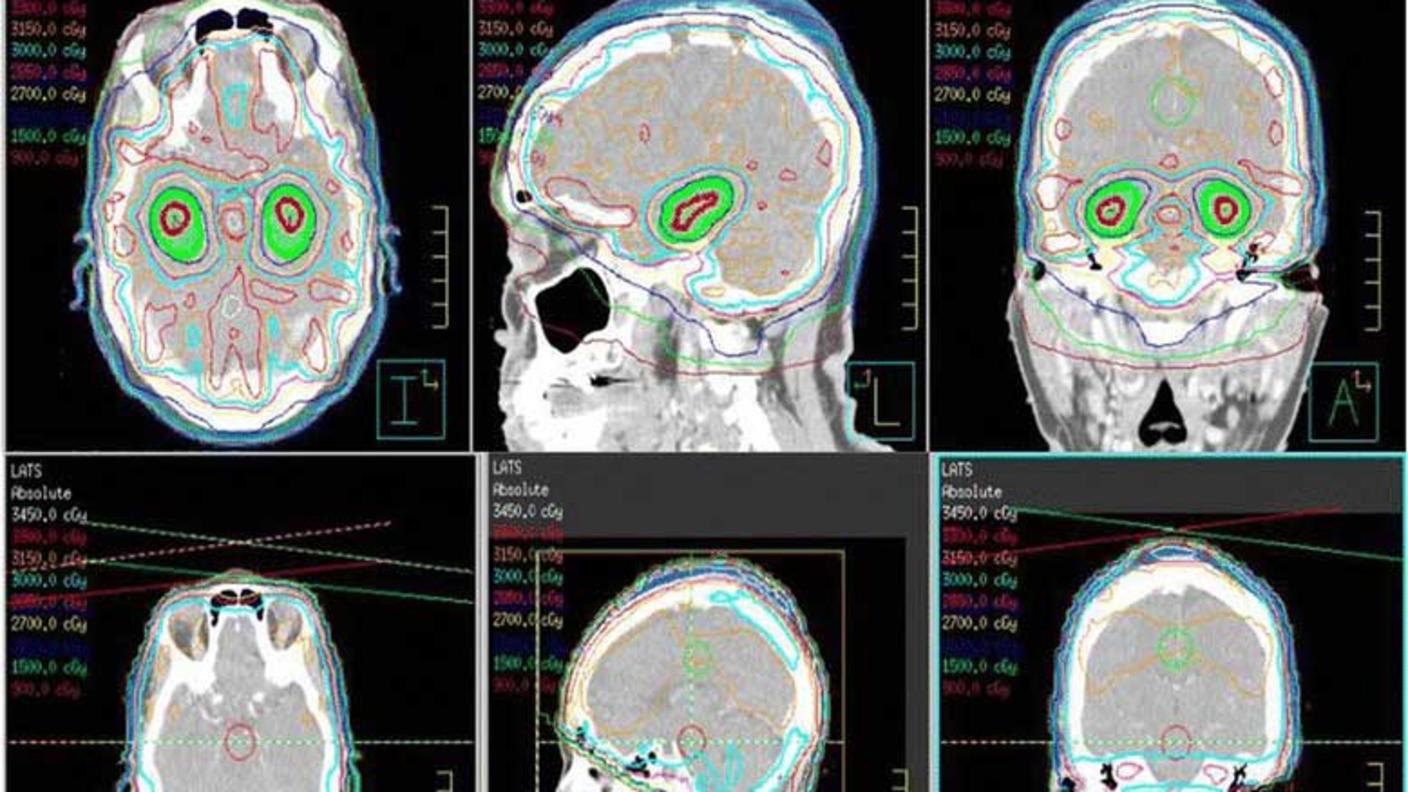
A classification system based primarily on how cells from a tumor biopsy look under the microscope has long been the standard tool for guiding meningioma treatment—in particular, determining whether someone should receive radiation therapy after surgery.
“Radiation therapy can work really well for controlling meningiomas,” said Dr. Raleigh. “But it can have significant long-term toxicities.” These can include secondary brain tumors and life-altering changes in cognition. “We really want to make sure that we’re offering this treatment only to the people whose tumors really need it.”
One method that researchers have been studying for potentially improving the standard decision-making approach is analyzing the activity of genes in meningiomas. That work has borne fruit, identifying many possible genes that may make a meningioma particularly problematic.
"But figuring out how all of those genes fit together was not something that had really been done before," said Dr. Raleigh.
Building and testing a predictive gene expression signature
For the study, the team, which was co-led by Stephen Magill, M.D., Ph.D., of Northwestern University and William Chen, M.D., also of UCSF, first analyzed the expression of genes in tumor samples from almost 180 people with meningiomas who had been treated at UCSF.
Starting with a much larger set of promising genes identified in previous studies, they winnowed them down to 34 that had significantly altered expression in samples depending on whether the tumors had grown slowly or quickly.
The researchers next tested the 34-gene signature in tumor samples taken from a separate group of 866 people with meningioma who had been treated at six different hospitals (called a validation group).
When they looked at which patients’ cancers came back and how quickly they came back, the signature accurately sorted people into groups at low, medium, or high risk of recurrence, both after initial surgery and after treatment for a meningioma that had already recurred. It also was better at predicting recurrence and survival than nine other classification systems it was tested against.
To date, only one prospective, randomized clinical trial has tested radiation therapy for the treatment of meningioma in North America. Using tumor samples collected during that trial, the researchers tested whether their score could pick out people who received radiation who may not have needed it, and people who didn’t receive it but likely should have.
As it did in the validation group, the score accurately predicted recurrence and survival in the 103 people who participated in that trial. Based on their gene expression scores, the researchers estimated that about 30% of people who had received radiation therapy were actually at low risk and likely hadn’t needed additional treatment immediately after surgery.
And tumors in almost 10% of people who didn’t receive radiation therapy in that trial likely needed it to prevent recurrence.
Dr. Raleigh and his colleagues are currently working with NRG Oncology, an NCI-funded clinical trials group, to test the signature in clinical trials that are under development to see if it can improve outcomes by guiding treatment.
“That’s something we’re very excited about,” he said.
The future of more-personalized meningioma treatment
Dr. Raleigh and his colleagues are continuing to study and better understand how to use this new gene expression signature.
This particular approach has one big advantage, Dr. Raleigh explained: The analysis of the genes can be performed using several common techniques in tumor samples preserved in different ways. So conducting the analysis needed to get the gene expression score is something that can be easily done across laboratories and hospitals.
In the future, the researchers would like to see if the 34-gene risk signature can potentially refine treatment even further—for example, identifying people whose tumors are so slow growing that they may not even need surgery up-front.
“About 1% of people will develop a meningioma during their lifetime, and it’s very common that these tumors are diagnosed incidentally, on brain imaging [done for other reasons],” Dr. Raleigh said. But “what we should do with those tumors … is not so clear.”
Currently, many people with meningiomas found in this way undergo regular imaging scans, putting off treatment unless the tumor begins to grow, Dr. Gilbert said.
If the 34-gene signature or a similar score can indicate which of these incidentally found tumors may never grow and which are destined to be fast growing, that could help further personalize treatment, Dr. Raleigh said.
In the past few years, several other classification systems have been developed that classify meningiomas based on potential therapeutic vulnerabilities, including one from Dr. Raleigh’s lab.
But it’s not a question of one system being better than the other, he explained.
“These systems provide complementary information,” he explained. The 34-gene expression score, for example, can help with decisions about currently used treatments. The system previously developed in Dr. Raleigh's lab, which is based on a type of change to DNA known as methylation, may help identify people who could participate in clinical trials of new targeted treatments.
This second score “sheds light on meningioma biology in terms of [tumor] drivers, and perhaps gives us a look into the future in terms of where we need to go with new therapies.”