Rare Melanoma Very Likely to Respond to Treatment with Pembrolizumab
, by Edward Winstead
People with desmoplastic melanoma, a rare form of skin cancer, are likely to benefit from treatment with a single immunotherapy drug, pembrolizumab (Keytruda), new results from a small clinical trial suggest.
Two combinations of immunotherapy drugs have been approved for melanoma. But the new findings suggest that people with desmoplastic melanoma could receive pembrolizumab alone and be spared the unnecessary side effects of additional therapies, according to the researchers who conducted the phase II clinical trial.
The trial included two parts and tested pembrolizumab in distinct ways: as a presurgical, or neoadjuvant, treatment in people with operable cancer and as an initial treatment for those who could not be treated with surgery.
Findings from the first part of the study, reported last year, indicate that neoadjuvant pembrolizumab may benefit people with desmoplastic melanoma that can be treated surgically.
The new results are from the second part of the trial, which included 27 people with inoperable metastatic desmoplastic melanoma. Among this group, 24 of 27 people (89%) responded to an initial treatment with pembrolizumab alone.
Nine patients (33%) had complete responses, meaning there was no evidence of their cancers after treatment, and 15 patients (55%) had their tumors shrink, known as partial responses.
Kari Kendra, M.D., Ph.D., of The Ohio State University Comprehensive Cancer Center presented the results at the American Association for Cancer Research annual meeting in Orlando, FL, on April 16.
The new findings will establish single-agent immunotherapy as the standard treatment for people with metastatic desmoplastic melanoma, predicted Vernon Sondak, M.D., who chairs the Department of Cutaneous Oncology at the Moffitt Cancer Center in Tampa, FL. He was not involved in the research presented in Orlando, although he helped lead the neoadjuvant desmoplastic melanoma study.
Both of the approved immunotherapy combinations to treat metastatic melanoma, nivolumab (Opdivo) plus ipilimumab (Yervoy) and relatlimab plus nivolumab (marketed under the name Opdualag), "are more toxic than single-agent pembrolizumab or nivolumab,” said Dr. Sondak.
Based on the new data, he continued, neither combination is likely to be the first choice for treating desmoplastic melanoma.
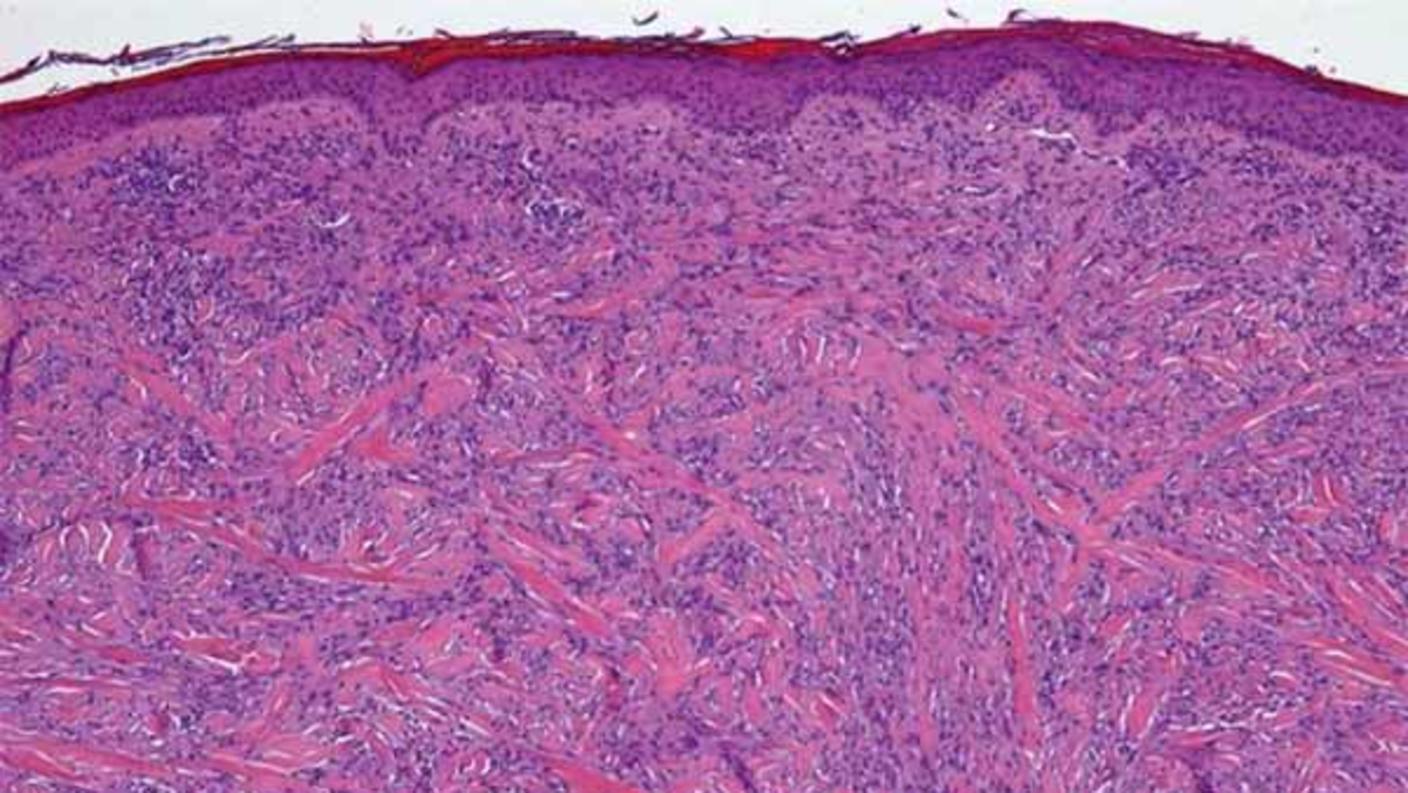
Desmoplastic melanomas have unusually large numbers of mutations
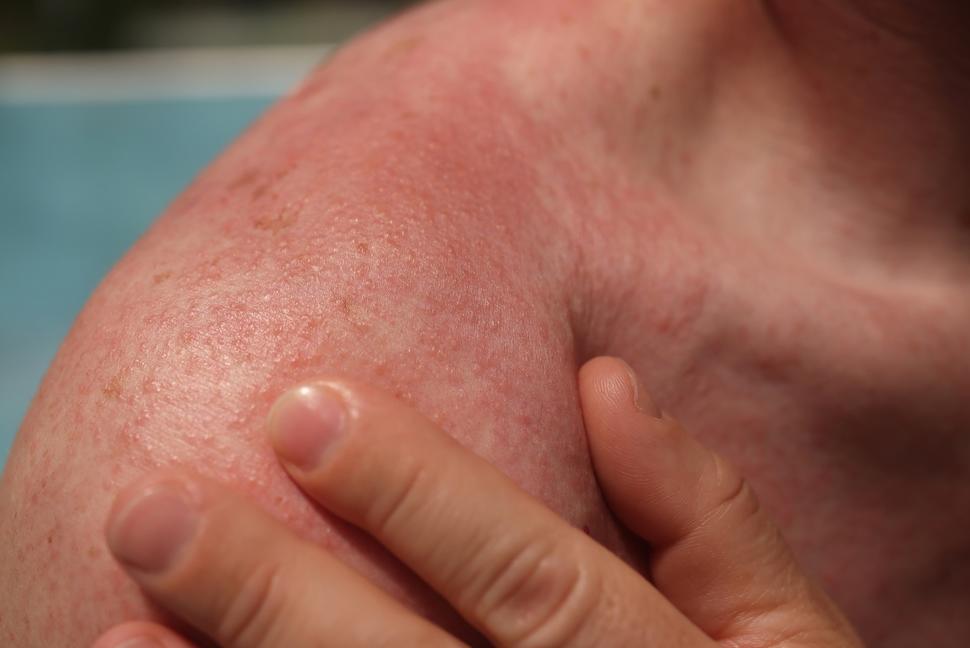
Desmoplastic melanoma is a rare disease that accounts for about 4% of the melanomas in the United States. The cancer, which occurs most often on the head and neck region, is caused by exposure to high levels of DNA-damaging ultraviolet radiation.
The tumors are often surrounded by thick layers of fibrous tissue and may affect nerves. Surgery to remove these tumors can be disfiguring, resulting in large scars on the head and neck.
Compared with other melanomas that arise on the skin, desmoplastic melanomas occur in older patients on more heavily sun-damaged areas. Most melanomas of the skin have a large number of genetic mutations caused by ultraviolet radiation, but desmoplastic melanomas tend to have even more mutations, noted Dr. Sondak.
“These mutations create [abnormal proteins] that the immune system can recognize and destroy when stimulated properly with immunotherapy,” Dr. Sondak said.
Confirming the results of a retrospective study
The new trial, called S1512, is the first to prospectively test a single immune checkpoint inhibitor in people with desmoplastic melanoma. NCI and Merck funded the study, which was conducted by the SWOG Cancer Research Network.
The findings reported at the AACR meeting confirm the results of an earlier retrospective study involving people with desmoplastic melanoma. In that study, pembrolizumab or nivolumab alone shrank tumors in 38 of 56 (68%) patients, and 19 patients (32%) had complete responses.
The overall response rate of nearly 90% in the current trial was impressive, Dr. Sondak said. “We simply haven’t seen response rates this high in any defined group of cancer patients treated with single agent immunotherapy, and in most cases not even with combination immunotherapy,” he added.
A possible exception, he noted, was a clinical trial testing a combination of two immunotherapy drugs (nivolumab plus ipilimumab) for metastatic Merkel cell carcinoma (another rare skin cancer). The trial had a 100% response rate in 22 patients who had not previously received immunotherapy.
“That exceptional result has not yet been validated outside of that trial, but it’s the only other example I know of in this category of extremely high immunotherapy response rates,” Dr. Sondak said.
Treatment options for patients with desmoplastic melanomas
Participants in the trial had their diagnoses confirmed by pathologists with experience evaluating desmoplastic melanomas.
“This study highlights the importance of an accurate diagnosis so that patients who have desmoplastic melanomas can learn about their treatment options,” said Elad Sharon, M.D., of NCI’s Cancer Therapy Evaluation Program, which helped support and monitor the trial. Immunotherapy drugs can have serious and even fatal side effects, he added.
In the new study, however, pembrolizumab alone was “very well tolerated by patients,” Dr. Sharon said. The most common side effects included fatigue, diarrhea, and rash.
A compelling story about science
The research leading up to the current trial began when clinicians observed some years ago that patients with desmoplastic melanoma seemed to be especially responsive to immunotherapy drugs. Meanwhile, molecular biologists who analyzed the genetic mutations in these tumors provided a rational explanation for why patients seemed to respond, Dr. Sondak said.
As next steps in the research, Dr. Kendra and her colleagues will continue to follow patients in the trial. They have also been studying the molecular features of tumors that progressed following treatment with pembrolizumab to try to learn why the cancers stopped responding to therapy.
A limitation of the study was its small size. Nevertheless, Dr. Sondak said, the trial results are “so compelling because they actually make sense based upon what we know about desmoplastic melanoma.”
“It’s a compelling story,” he continued, “and a great demonstration of what clinicians and scientists can do together to make meaningful improvements in the treatment of our patients.”