Trial Results Support Adding Daratumumab to Initial Treatment for Multiple Myeloma
, by Nadia Jaber
A large randomized clinical trial for people newly diagnosed with multiple myeloma has found that adding the drug daratumumab (Darzalex) to a standard treatment regimen was more effective than that standard regimen on its own.
Trial participants treated with daratumumab lived substantially longer without their cancer getting worse or dying than those who received the standard treatment only.
After a median of 4 years, 84% of those who received daratumumab plus the standard treatment and 68% of patients who received the standard treatment—which included bortezomib (Velcade), lenalidomide (Revlimid), and dexamethasone—were alive and free of cancer progression.
That is “a striking benefit in progression-free survival,” said C. Ola Landgren, M.D., Ph.D., director of the Sylvester Myeloma Institute at the University of Miami, who was not involved in the trial.
People treated with daratumumab were also more likely to have no detectable signs of cancer (what’s called minimal residual disease, or MRD) after treatment. Findings from the trial, called PERSEUS, were published in the New England Journal of Medicine and presented at the annual meeting of the American Society of Hematology on December 12.
In 2020, a small clinical trial found that adding daratumumab to this standard treatment regimen resulted in substantial benefits for people with newly diagnosed multiple myeloma.
While some physicians started prescribing this drug combination right away, others have been holding out for stronger data, explained Surbhi Sidana, M.D., a hematologist–oncologist at Stanford University.
Results from the new trial provide “very important confirmatory data that settles this issue for people who were holding out on progression-free survival [data] from a phase 3 study. For people who were waiting, this changes their practice,” Dr. Sidana said at the annual meeting of the American Society of Hematology.
While daratumumab is approved by the Food and Drug Administration for use with other drug combinations, it is not yet approved for use with bortezomib, lenalidomide, and dexamethasone for newly diagnosed multiple myeloma.
Intravenous or subcutaneous daratumumab
For many younger people who have just been diagnosed with multiple myeloma, the initial treatment is a multistep process that includes a cocktail of drugs and a stem cell transplant.
This approach has been in place for more than 10 years, noted Pieter Sonneveld, M.D., Ph.D., of Erasmus MC Cancer Institute in the Netherlands and leader of the PERSEUS trial.
Over the past decade, researchers have been tweaking the drug cocktail, swapping out or adding in new medicines to see if different combinations better eradicate the cancer and delay its return.
“With the PERSEUS study, we tried to improve on the standard treatment by adding daratumumab,” Dr. Sonneveld said.
Daratumumab is a targeted cancer drug that kills multiple myeloma cells and also recruits immune cells to help kill cancer cells. It can be infused through a vein (intravenous) or injected under the skin (subcutaneous).
Whereas both delivery routes have similar effects on multiple myeloma, subcutaneous injections of daratumumab cause fewer adverse reactions and are far less of a time burden for patients. Intravenous infusions are given over the course of a few hours, while subcutaneous injections take only a few minutes, Dr. Landgren explained.
In the 2020 trial, daratumumab was given through intravenous infusions. But since then, subcutaneous injections have been preferred, Dr. Landgren said. In the PERSEUS trial, the researchers used subcutaneous daratumumab.
The PERSEUS trial
More than 700 people with newly diagnosed multiple myeloma participated in the trial, which took place across 14 countries in Europe and Australia and was partially funded by Janssen, the company that makes daratumumab.
All participants were eligible for a stem cell transplant, which typically means they are younger and have better overall health. All participants were randomly assigned to receive the following standard regimen with or without subcutaneous daratumumab:
- Induction therapy with bortezomib, lenalidomide, and dexamethasone
- Stem cell transplant
- Consolidation therapy with bortezomib, lenalidomide, and dexamethasone
- Maintenance therapy with lenalidomide
Patients in the daratumumab group received the drug as part of induction, consolidation, and maintenance therapy.
Investigators tracked patients’ outcomes using multiple measures for a median of nearly 4 years. For all measures, the standard regimen plus daratumumab was more effective than the standard regimen alone.
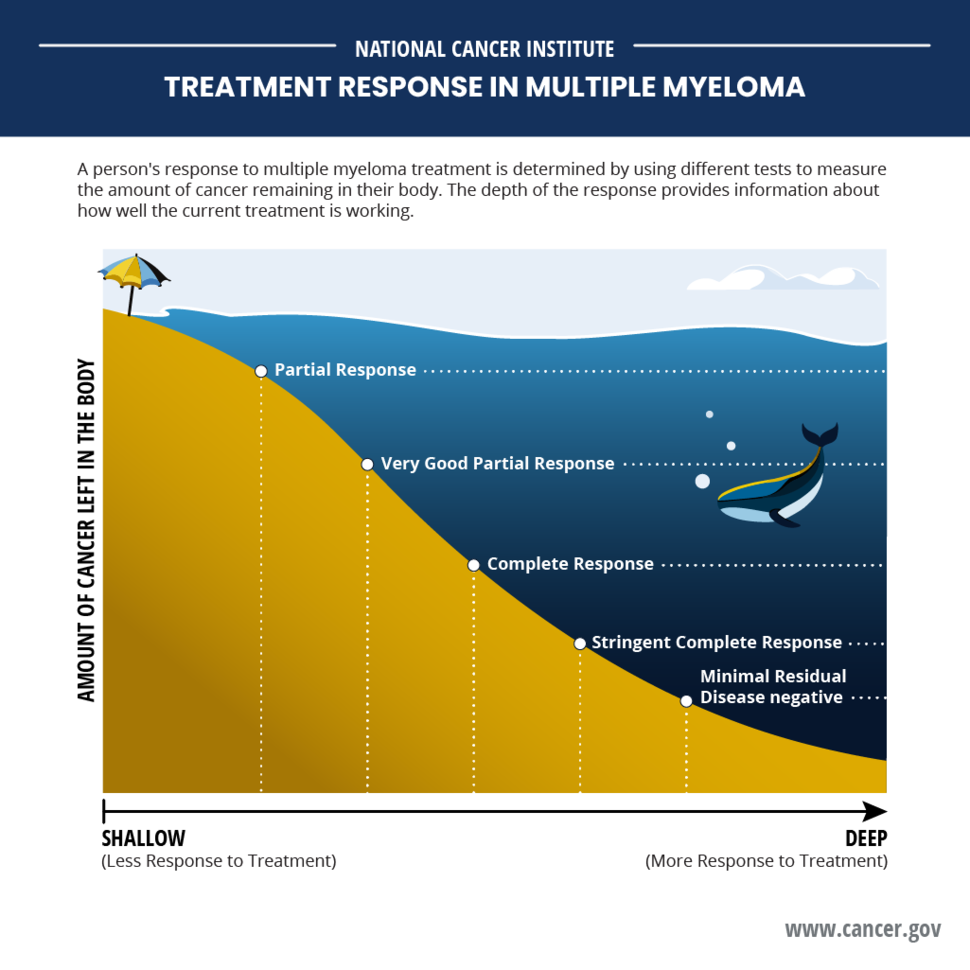
For example, people in the daratumumab group had higher rates of deep responses, such as complete or stringent complete responses (no signs of cancer using classic blood and other tests), and MRD negativity (no signs of cancer using highly sensitive DNA tests).
| Daratumumab plus standard treatment |
Standard treatment | |
| Progression-free survival at 4 years | 84% | 68% |
| Complete response or stringent complete response | 88% | 70% |
| MRD negative | 75% | 48% |
| MRD negative for at least 1 year | 65% | 30% |
People in the daratumumab group who stayed MRD negative for at least a year were able to stop taking daratumumab as maintenance therapy and remained cancer free. That’s important, Dr. Sonneveld said, because taking fewer drugs long-term for maintenance therapy often translates to a better well-being and quality of life.
Adding daratumumab to the standard treatment resulted in a nearly 60% drop in the risk of cancer progression or death (hazard ratio of 0.42), the researchers determined.
The magnitude of that change is “unprecedented in these kinds of phase 3 trials [for] multiple myeloma,” Dr. Sonneveld said.
Participants have not been followed long enough to see if daratumumab improved how long they live overall. The trial is ongoing and overall survival will continue to be tracked, the investigators noted.
Side effects of adding daratumumab
Side effects like infections and a lower-than-normal number of white blood cells (neutropenia) or platelets (thrombocytopenia) were more common among patients in the daratumumab group.
A higher percentage of patients in the daratumumab group than the standard treatment group experienced a serious side effect (57% versus 49%). However, fewer patients in the daratumumab group stopped treatment due to side effects (9% versus 22%).
Eleven percent of patients in the daratumumab group and 7% in the standard treatment group developed a second type of cancer—meaning, cancer that was not multiple myeloma. Slightly more patients in the daratumumab group than the standard treatment group were able to receive a stem cell transplant (90% versus 87%). A serious side effect of stem cell transplantation is the risk of additional cancers.
A similar percentage of patients in both groups died from complications related to side effects: 4% in the daratumumab group and 5% in the standard treatment group.
One among several treatment options
Findings from the PERSEUS trial will likely add one more option to the treatments available for people with multiple myeloma, Dr. Landgren said.
Daratumumab is already FDA approved to be used with other drug combinations for newly diagnosed multiple myeloma. One such combination includes bortezomib, dexamethasone, and thalidomide (Thalomid).
And other options may be coming down the line. The ongoing ADVANCE study is comparing daratumumab plus another three-drug combination (carfilzomib [Kyprolis], lenalidomide, and dexamethasone) to the three-drug combination alone in people with newly diagnosed multiple myeloma.
And another clinical trial is investigating the effects of adding isatuximab (Sarclisa)—which targets the same protein as daratumumab—to carfilzomib, lenalidomide, and dexamethasone. Preliminary results of the trial, also presented at the American Society of Hematology annual meeting, suggest that the combination is promising for newly diagnosed multiple myeloma.
What’s currently unclear is whether patients need to take daratumumab during all pre- and post-transplant phases of therapy to reap its benefits, Dr. Landgren said.
One ongoing clinical trial is exploring that question. In this trial, daratumumab was added to bortezomib, thalidomide, and dexamethasone. Early results from the trial suggest that daratumumab may be most effective when added to all three phases of the standard treatment regimen.