December 2017 - Cancer Currents Blog
-
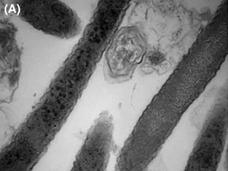
Fusobacterium May Help Colorectal Cancer Grow and Spread
Fusobacterium, found in the stomach and intestines, may help fuel the growth of colorectal cancer and metastases. In a mouse model of colorectal cancer, using antibiotics to kill these bacteria slowed tumor growth.
-
New on NCI’s Websites for December 2017
NCI periodically provides updates on new websites and other online content of interest to the cancer community.
-
Genomic Profiling Tests Cleared by FDA Can Help Guide Cancer Treatment, Clinical Trial Enrollment
The FDA has recently approved two tests to identify genetic alterations in tumors. One of the tests can be used to identify patients who may be candidates to receive specific targeted therapies.
-
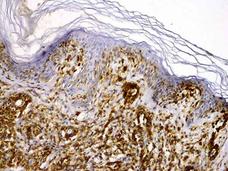
Brentuximab Vedotin Approved for Two Rare Lymphomas
FDA has approved brentuximab vedotin (Adcetris®) for the treatment of adults who have been treated previously for either primary cutaneous anaplastic large cell lymphoma or CD30-expressing mycosis fungoides, two rare lymphomas that start as rashes on the skin.
-
NCI: Taking Risks to Advance Science
Norman Sharpless, M.D., offers his thoughts on his experience thus far as NCI director and areas, like big data, where NCI can play an important role in advancing science.
-
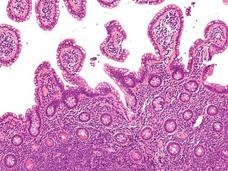
Prior Cancers Common in Patients Newly Diagnosed with Cancer
A new study shows that many patients diagnosed with a new cancer have had one or more cancers in the past, which has potential implications for long-term surveillance and clinical trial enrollment.
-
Acalabrutinib Receives FDA Approval for Mantle Cell Lymphoma
The FDA has granted accelerated approval to acalabrutinib (Calquence®) for the treatment of adults with mantle cell lymphoma whose cancer has progressed after receiving at least one prior therapy.
-
With Advances in Cancer Immunotherapy, Scientists Discuss Need to Develop New Mouse Models
A recent NCI symposium focused on developing new and better mouse models for testing treatments that harness the immune system against cancer.
-
FDA Approves Alectinib For Initial Treatment of ALK-Positive Lung Cancer
FDA has approved alectinib (Alecensa) as a first-line treatment option for patients with advanced non-small cell lung cancer that is ALK positive. Alectinib is the third ALK inhibitor to be approved in this setting.
-
Fat Cells May Hinder Effectiveness of Chemotherapy
Researchers have shown that fat cells can absorb two commonly used chemotherapy drugs and break them down chemically into a less toxic form, potentially reducing the drugs’ effectiveness.
-
Expanding Cancer Clinical Trial Access for Patients with HIV
People with HIV are often excluded from clinical trials to protect their safety. Preliminary results from an NCI-sponsored study of an immunotherapy drug show that people with HIV can safely participate in clinical trials.