Treatment - Cancer Currents Blog
Cancer treatment related news, with context from leading experts. Includes articles on new therapies, treatment side effects, and important trends in treatment-related research.
-
FDA Approval Likely to Change Initial Treatment for Some People with Advanced Colorectal Cancer
FDA has approved the combination of the immunotherapy drugs ipilimumab (Yervoy) and nivolumab (Opdivo) for the initial treatment of people with advanced colorectal cancer whose tumors are classified as MSI-H or dMMR.
-
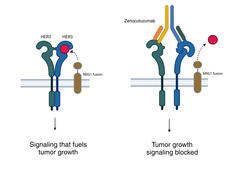
Zenocutuzumab Approved to Treat Lung and Pancreatic Cancers with Rare Genetic Change
FDA has approved zenocutuzumab (Bizengri) to treat people with pancreatic or non-small cell lung cancer whose tumors have a rare genetic alteration called an NRG1 fusion. The approval is based on a clinical trial in which the drug shrank tumors in a third of patients.
-
Combination Therapy for Metastatic Prostate Cancer Helps Some Men Live Longer
For men with metastatic castration-resistant prostate cancer, initial treatment with enzalutamide (Xtandi) combined with talazoparib (Talzenna) may help them live longer than getting enzalutamide alone, according to updated results from a large clinical trial.
-
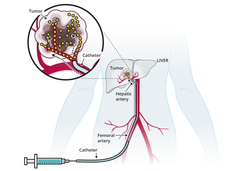
TACE-Based Treatment Combinations Effective Against Intermediate-Stage Liver Cancer
Results from two clinical trials show that combining a procedure called TACE with an immunotherapy drug and angiogenesis inhibitor improves how long people with intermediate-stage liver cancer live without their disease returning or getting worse.
-
FDA Approves Injectable Nivolumab, an Alternative to IV Infusion
The injectable form of nivolumab, called Opdivo Qvantig, is quicker and easier to give, several oncologists said, and is just as effective as the intravenous form. Injectable forms of other immunotherapies are also on the horizon.
-
Many Men with Metastatic Prostate Cancer Are Not Getting the Recommended Treatments, Study Finds
Many U.S. doctors aren’t using the recommended initial treatments for their patients with hormone-sensitive metastatic prostate cancer, a new study has found. Often, it’s because they aren’t up to date on the latest treatment recommendations or because of side effect concerns.
-
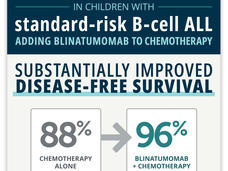
Blinatumomab Boosts Chemotherapy as Initial Treatment for Some Kids with ALL
Following positive results from a clinical trial, the immunotherapy drug blinatumomab (Blincyto) is expected to become part of the standard initial treatment for many kids with B-cell acute lymphoblastic leukemia, the most common form of childhood cancer.
-
FDA Approvals Expand Initial Treatment Options for Multiple Myeloma
FDA’s approvals of Darzalex Faspro and Sarclisa, each used in combination with standard three-drug treatment regimens, should change the initial treatment of newly diagnosed multiple myeloma, including for patients who can’t get a stem cell transplant.
-
Experimental CAR T-Cell Therapy Shrinks Tumors in Children with Deadly Brain Cancer
In a small clinical trial, an experimental CAR T-cell therapy that targets the protein GD2 on cancer cells shrank tumors—for 2 years or more in several cases—in children and young adults with diffuse midline glioma, an aggressive brain and spinal cord cancer.
-
Cetuximab Outperforms Durvalumab for Head and Neck Cancer When Cisplatin Isn’t an Option
The findings from a recent NCI-supported clinical trial are helpful because previous studies for people with locally advanced head and neck cancer have yielded conflicting data for and against several alternatives to cisplatin combined with radiation.
-
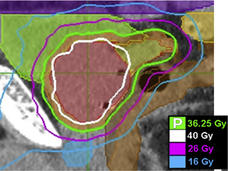
Trial Results Support SBRT as a Standard Option for Some Prostate Cancers
In a trial, men who received stereotactic body radiotherapy (SBRT) didn’t have a higher risk of cancer recurrence than men treated with other common radiation therapy regimens that are given over longer periods.
-
Nivolumab Appears to Boost Cure Rate in Advanced Hodgkin Lymphoma
In a nearly 1,000-patient trial, treatment with nivolumab (Opdivo) and the chemotherapy regimen AVD was better at eliminating cancer and keeping it at bay than the current standard initial treatment for the disease, AVD and brentuximab (Adcetris). The nivolumab combination also had fewer side effects.
-
Bladder Cancer Trial Finds Extended Lymph Node Surgery Doesn’t Improve Survival
A randomized clinical trial comparing two types of surgery in people with localized muscle-invasive bladder cancer found that more extensive surgery removing a larger group of lymph nodes did not improve survival, compared with standard lymph node surgery.
-
Combination Chemo Helps People with Leiomyosarcoma Live Longer
Results from a French clinical trial have identified what experts say should now be the recommended initial treatment of advanced leiomyosarcoma. In the trial, the combination of trabectedin (Yondelis) and doxorubicin improved survival by a median of 9 months.
-
Jaw Problems Linked to Bone-Modifying Drugs Not as Rare as Once Thought
Osteonecrosis of the jaw was thought to be a rare side effect of drugs like denosumab (Xgeva) that lessen bone problems when cancer has spread to the bone. But a new study has found that the painful side effect is more common than once thought.
-
Clinical Trial Results Support Uninterrupted Use of Imatinib for Some Gastrointestinal Stromal Tumors
Trial participants who stopped imatinib had a more rapid worsening of disease, a shorter time until resistance, and did not live as long as participants who continued the therapy uninterrupted.
-
Understanding the Risk of Second Cancers After CAR T-Cell Therapy
In late 2023, FDA announced it was investigating instances of second cancers following treatment with CAR T-cell therapies. In this Q&A, NCI’s Dr. Stephanie Goff explains what’s known about the issue, stressing that second cancers “of any kind are rare.”
-
More Immunotherapy Options Approved for Treating Endometrial Cancer
People with advanced endometrial cancer now have new FDA-approved treatment options: pembrolizumab and durvalumab, paired with chemotherapy, for tumors with a genetic change called mismatch repair deficiency. The agency also expanded the approved uses of dostarlimab for the disease.
-
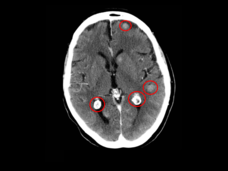
Lorlatinib Slows Growth of ALK-Positive Lung Cancers, May Prevent Brain Metastases
Lorlatinib (Lorbrena) is superior to crizotinib (Xalkori) as an initial treatment for people with ALK-positive advanced non-small cell lung cancer, according to new clinical trial results. Treatment with lorlatinib also helped prevent new brain metastases.
-
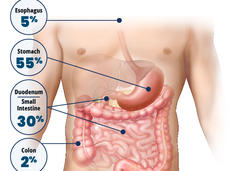
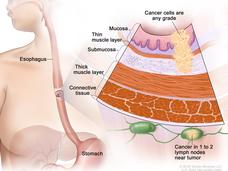
Trial Establishes Preferred Treatment for Some People with Esophageal Cancer
For people with locally advanced esophageal cancer, a chemotherapy regimen called FLOT is the preferred treatment, according to results from a large clinical trial. People treated with FLOT lived much longer than those treated with the CROSS regimen.