Combo of Ribociclib, Letrozole Improves Survival in Advanced Breast Cancer
, by Carmen Phillips
Updated results from a large study have further cemented the role of drugs called CDK4/6 inhibitors in treating people with the most common form of metastatic breast cancer.
In the clinical trial, called MONALEESA-2, women treated with the CDK4/6 inhibitor ribociclib (Kisqali) and the hormone-blocking drug letrozole (Femara) as their initial treatment for advanced breast cancer lived approximately 1 year longer overall than women treated with letrozole alone.
The median overall survival was nearly 64 months in patients treated with both drugs and over 51 months in those who only received letrozole. All the women in the trial had already gone through menopause and had cancer that was hormone receptor (HR)-positive and HER2-negative.
“This is the longest median survival reported to date in any advanced breast cancer phase 3 clinical trial,” said the study’s lead researcher, Gabriel Hortobagyi, M.D., of the University of Texas MD Anderson Cancer Center. Dr. Hortobagyi presented the findings on September 18 at the 2021 European Society of Medical Oncology (ESMO) Congress.
In addition, more than half of the trial participants treated with ribociclib and letrozole were still alive 5 years after beginning treatment. That is a first for a study involving people with advanced breast cancer, he said.
Ribociclib is already approved by the Food and Drug Administration as an initial, or first-line, treatment (with an aromatase inhibitor, like letrozole) for postmenopausal women with HR-positive, HER2-negative advanced breast cancer. That approval was based on earlier results from the MONALEESA-2 trial that showed patients treated with ribociclib and letrozole lived longer without their cancer getting worse (progression-free survival), compared with those treated with letrozole alone.
Two other CDK4/6 inhibitors—palbociclib (Ibrance) and abemaciclib (Verzenio)—are also approved for the treatment of people with this same form of breast cancer, both based on large clinical trials in which the drugs were shown to improve progression-free survival.
A CDK4/6 inhibitor combined with a hormone-blocking drug is now the standard first-line treatment for postmenopausal women with this form of advanced breast cancer, explained Stanley Lipkowitz, M.D., Ph.D., chief of the Women’s Malignancies Branch in NCI’s Center for Cancer Research.
Now that there’s evidence that ribociclib improves how long patients live overall, “it raises the question of whether it should be the preferred CDK4/6 inhibitor for most of these patients,” Dr. Lipkowitz said.
Adding CDK4/6 Inhibitors to Endocrine Therapy
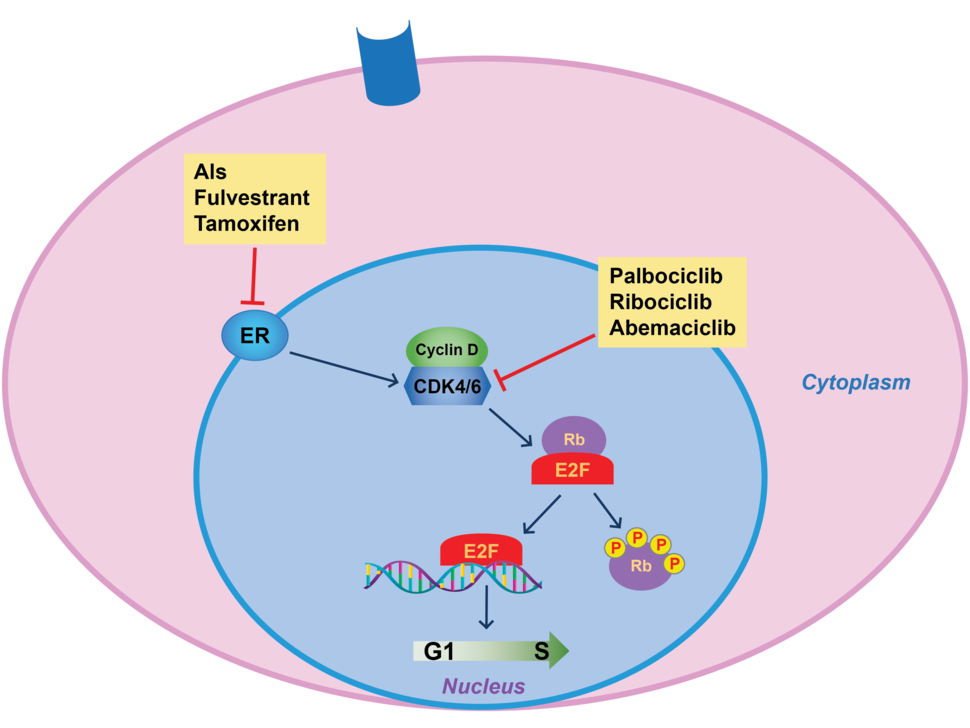
As their name implies, CDK4/6 inhibitors work by blocking the activity of two enzymes, CDK4 and CDK6, that help control cell division. Both enzymes are commonly found at higher-than-normal amounts in breast cancer cells, particularly those that overproduce hormone receptors.
For many years, treatment of HR-positive, HER2-negative advanced breast cancer was centered around therapies that blunt estrogen’s ability to fuel cancer—also called endocrine therapy. Those treatments include aromatase inhibitors, tamoxifen, and fulvestrant (Faslodex).
Although these treatments can be very effective, they eventually stop working in most patients.
The emergence of CDK4/6 inhibitors in the mid-2010s altered the treatment landscape. Clinical trials showed that combining CDK4/6 inhibitors with estrogen-blocking drugs could substantially lengthen the amount of time it took for patients’ tumors to become resistant to treatment, regardless of their menopause status.
Currently, there is general agreement among oncologists who specialize in treating breast cancer that the three CDK4/6 inhibitors, all of which are taken as a pill, work equally well. But multiple factors can influence oncologists’ choice of which drug to use for which patients, said Melissa McShane, M.D., of the Breast Cancer Oncology Program at Fox Chase Cancer Center. Side effects are an important one.
For example, a low level of white blood cells (neutropenia) is a common side effect of ribociclib and palbociclib, Dr. McShane explained. Neutropenia can lead to fevers and rashes, among other symptoms, and leave patients vulnerable to infections. So, for patients who are unable to visit a facility to have their blood levels checked regularly, oncologists might prescribe abemaciclib.
However, abemaciclib is much more likely to cause diarrhea, which might be particularly problematic for patients with certain other health conditions.
So, when it comes to selecting a CDK4/6 inhibitor, “it really depends on the patient,” she said.
For most physicians, a key consideration for choosing a treatment is whether it will help their patients live longer, said Howard A. “Skip” Burris, M.D., of Sarah Cannon Research Institute at Tennessee Oncology, and a researcher on the MONALEESA-2 trial.
And until now, Dr. Burris said, no trial of any one of these drugs had proved they could do that.
Longest Survival in Advanced Breast Cancer Ever Reported
MONALEESA-2 enrolled 668 participants who were randomly assigned to initial treatment with ribociclib and letrozole or a placebo and letrozole. Nearly all participants had at least one confirmed metastatic tumor. The trial was funded by Novartis, the company that makes ribociclib.
Participants continued taking their assigned treatment until their cancer started to get worse—at which point most received other treatments in consultation with their oncologists—or until they had side effects that made it too difficult to continue the treatment.
The first follow-up results from the trial showed that participants treated with ribociclib and letrozole lived substantially longer without their disease getting worse than those treated with letrozole alone.
With longer follow-up, there was a 12.5-month improvement in median overall survival, Dr. Hortobagyi reported at the ESMO meeting. In addition, 52% of those in the ribociclib group were alive 5 years after beginning treatment, compared with 44% in the letrozole-only group.
| Treatment | Progression-free survival (median) | Overall survival (median) | Trial participants alive at 5 years |
|---|---|---|---|
| Ribociclib + letrozole | 25.3 months | 63.9 months | 52% |
| Letrozole + placebo | 16.0 months | 51.4 months | 44% |
More than half of patients in the ribociclib group remained on the drug for at least 2 years, Dr. Hortobagyi reported.
When patients’ cancers start to get worse during initial treatment, they will often get chemotherapy as part of their next line of treatment. But in the MONALEESA-2 trial, Dr. Hortobagyi reported, patients in the ribociclib group were able to avoid taking chemotherapy for up to a year or longer than those in the letrozole-only group.
More than one-third of patients in the letrozole-only treatment group eventually went on to receive a different CDK4/6 inhibitor, most often palbociclib, after their cancer began to progress.
Patients tend to tolerate the side effects of ribociclib very well, Dr. Burris said. Even so, in the MONALEESA-2 trial, about 11% of participants taking ribociclib stopped taking the drug at some point because of side effects, compared with about 3% among in the letrozole-only group.
One concern with CDK4/6 inhibitors has been their link to a heart rhythm condition called long QT syndrome. Less than 5% of patients taking ribociclib experienced this issue, Dr. Hortobagyi reported, and none had any clinical problems associated with it.
Overall, he said, many of the side effects considered to be potentially serious, including neutropenia, did not cause symptoms and were “completely reversible.”
Using CDK4/6 Inhibitors as a First-Line Treatment
Some oncologists still often reserve CDK4/6 inhibitors as a second-line treatment for their patients with advanced breast cancer, waiting until the disease has started to progress on hormone-blocking therapies. But Dr. Burris said he hopes this thinking will now change.
“We shouldn’t be ‘saving’ these therapies,” Dr. Burris said. “Regardless of which [drug] you pick, oncologists should be offering patients these drugs as a first-line treatment.”
There doesn’t appear to be a consensus on whether the updated findings from MONALEESA-2 mean ribociclib should be the preferred initial treatment.
Dr. Burris pointed to similar overall survival improvements seen with ribociclib in two other large clinical trials involving people with advanced breast cancer: MONALEESA-3 (in combination with fulvestrant) and MONALEESA-7, in younger, premenopausal patients.
Because of his long involvement in clinical trials of ribociclib and the consistency of the survival data from these trials, Dr. Burris said that he tends to favor it for many of his patients.
Other oncologists are likely to take the MONALEESA-2 results as confirmation that any of the CDK4/6 inhibitors are a good choice, Dr. McShane said.
Overall survival data from large clinical trials of palbociclib and abemaciclib as a first-line treatment in postmenopausal women with HR-positive, HER2-negative advanced breast cancer (called PALOMA-2 and MONARCH 3, respectively) are expected to be available soon, Dr. McShane said, and she expects that they will also show an improved overall survival with the CDK4/6 inhibitor.
Nevertheless, she continued, the MONALEESA-2 data are important.
“We’ve been anticipating these results and it’s exciting to see them,” Dr. McShane said. “They correlate with what we’re seeing in the ‘real world.’ And patients are always asking for numbers [on survival], and now we can now give them those numbers.”